

热处理时间对锂电池正极材料Cr8O21的影响
收稿日期: 2021-01-25
修回日期: 2021-04-14
网络出版日期: 2021-05-04
基金资助
贵州省科技计划项目(20202Y057)
Influence of Heat Treatment Time on Cathode Material Cr8O21 for Lithium Battery
Received date: 2021-01-25
Revised date: 2021-04-14
Online published: 2021-05-04
滕久康 , 王庆杰 , 张亮 , 张红梅 , 陈晓涛 , 张鹏 , 赵金保 . 热处理时间对锂电池正极材料Cr8O21的影响[J]. 电化学, 2021 , 27(6) : 689 -697 . DOI: 10.13208/j.electrochem.210121
Chromium oxide (Cr8O21) cathode material for lithium batteries was synthesized by thermal decomposition of chromium trioxide (CrO3) at high temperature. The electrochemical properties of chromium oxide depended on the time and temperature during the heat treatment. Pure phase chromium oxide was prepared, and the effects of heat treatment time on the structures and electrochemical properties of Cr8O21 were systematically studied. The first discharge mechanism of chromium oxide in lithium batteries was explored, and the results were similar to that in lithium-sulfur batteries. The crystal phases and electrochemical properties of the prepared chromium oxide were analyzed by TGA, XRD, SEM, EDS, ICP, EIS techniques and constant current discharge measurement. The results show that heat treatment time had an important impact. Extending the heat treatment time was beneficial to improve the electrochemical properties of the material. The less the amount of residual CrO3, the better the electrochemical performance. The severe oxidation reaction between CrO3 and the electrolyte caused the electrode to be corroded. The material obtained in 48 h exhibited excellent performance, complete crystallization, good morphology, and low electrochemical impedance. At a constant discharge current of 0.05 mA, the specific capacity of the material reached 383.26 mAh·g-1 with the specific energy of 1153.83 mWh·g-1 and the average discharge voltage of 3.01 V. This study provides an effective way to prepare pure phase chromium oxide and proves its potential application in the field of lithium batteries.
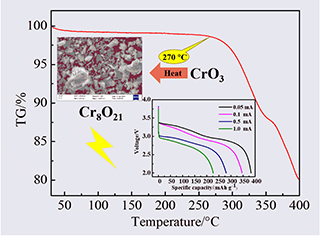
Key words: lithium battery; cathode material; Cr8O21; chromium oxide; heat treatment time
| [1] | Yuan Z Z(袁中直), Liu J C(刘金成), Lü Z Z(吕正中), Zhu Y(祝媛), Pu F(卜芳). Technical progress and future of lithium primary batteries[J]. J. Power Sources (Chinese)(电源技术), 2019, 5(43): 735-738. |
| [2] | Besenhard J O, Schollhorn R. Chromium oxides as cathodes for secondary high energy density lithium batteries[J]. J. Electrochem. Soc., 1977, 124(7): 968-971. |
| [3] | Takeda Y, Kanno R, Tsuji Y, Yamamoto O, Taguch H. Chromium oxides as cathodes for lithium cells[J]. J. Power Sources, 1983, 9: 325-328. |
| [4] | Li T(李婷), Qian J F(钱江峰), Cao Y L(曹余良), Yang H X(杨汉西), Ai X P(艾新平). Electrochemical performance of Li1-xMxFePO4 cathode materials synthesized by polymer pyrolysis route[J]. J. Electrochem.(电化学), 2007, 13(2): 136-139. |
| [5] | Xiao J(肖婕), Zhan H(詹晖), Zhou Y H(周运鸿). Synjournal and electrochemical behavior of layered-structure LiMn1-xCrxO2[J]. J. Electrochem.(电化学), 2004, 10(3): 324-329. |
| [6] | Desilvestro J, Haas O. Metal oxide cathode materials for electrochemical energy storage: a review[J]. J. Electrochem. Soc., 1990, 137(1): 5C-22C. |
| [7] | Liu J Y(刘建勇), Li H(李泓), Wang Z X(王兆翔), Huang X J(黄学杰). Study on preparation and performance of chromium oxide cathode material for secondary lithium battery[A]. Soild State Ionics, 2004: 57-59. |
| [8] | Feng X Y, Ding N, Wang L, Ma X H, Li Y M, Chen C H. Synjournal and reversible lithium storage of Cr2O5 as a new high energy density cathode material for rechargeable lithium batteries[J]. J. Power Sources, 2013, 222: 184-187. |
| [9] | Ramaraja P R, Ramadass P, Bala S H, Branko N P. Synjournal, characterization and cycling performance of novel chromium oxide cathode materials for lithium batteries[J]. J. Power Sources, 2003, 124: 155-162. |
| [10] | Liu J Y, Wang Z X, Li H, Huang X J. Synjournal and characterization of Cr8O21 as cathode material for rechargeable lithium batteries[J]. Solid State Ionics, 2006, 177: 2675-2678. |
| [11] | Liu D X(刘东旭). Preparation and electrochemical performance studies of chromium oxide as cathode materials for lithium batteries[D]. Harbin: Harbin Institute of Technology(哈尔滨工业大学), 2019. |
| [12] | Yamamoto O, Takeda Y, Kanno R, Oyabe Y, Shinya Y. Amorphous chromium oxide; a new lithium battery cathode[J]. J. Power Sources, 1987, 20(1): 151-156. |
| [13] | Feng G X, Li L F, Liu J Y, Liu N, Li H, Yang X Q, Huang X J, Chen L Q. Enhanced electrochemical lithium storage activity of LiCrO2 by size effect[J]. J. Mater. Chem., 2009, 19: 2993-2998. |
| [14] | Zhuang Q C(庄全超), Xu S D(徐守冬), Qiu X Y(邱祥云), Cui Y L(崔永丽), Fang L(方亮), Sun S G(孙世刚). Diagnosis of electrochemical impedance spectroscopy in lithium ion batteries[J]. Prog. Chem.(化学进展), 2010, 22(6): 1044-1057. |
/
| 〈 |
|
〉 |