

纳米晶枝CuAu 合金催化剂对二氧化碳电催化还原性能的研究
收稿日期: 2021-02-22
修回日期: 2021-04-16
网络出版日期: 2021-04-10
Synthesis and Electrochemical Study of CuAu Nanodendrites for CO2 Reduction
Received date: 2021-02-22
Revised date: 2021-04-16
Online published: 2021-04-10
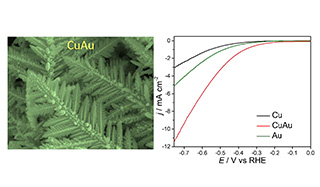
利用可再生清洁能源将CO2转化为CO和其他小分子是合成含碳燃料的可观方法之一。间歇性可再生能源存储的重要策略之一是将二氧化碳进行电化学还原。选择具有高活性和稳定性的电催化剂对于电化学还原CO2至关重要。在这项研究中,我们使用简单的电沉积方法合成了具有纳米晶枝状结构的CuAu合金电极。各项表征显示原子比约为1:1的CuAu纳米枝晶对CO2的电化学还原具有出色的催化活性。合成的主要产物是H2和CO,这是合成气体是合成天然气,氨和甲醇合成的中间体。电化学阻抗谱(EIS)测量表明,相对于Cu和Au电沉积催化剂,CuAu纳米晶枝状催化剂具有相对低的电荷转移阻力。CuAu纳米枝晶催化剂是一种具有潜在的转化CO2为合成气体的高活性电催化剂。
Dylan Siltamaki , 陈帅 , Farnood Pakravan , Jacek Lipkowski , 陈爱成 . 纳米晶枝CuAu 合金催化剂对二氧化碳电催化还原性能的研究[J]. 电化学, 2021 , 27(3) : 278 -290 . DOI: 10.13208/j.electrochem.201253
The conversion of carbon dioxide (CO2) to carbon monoxide (CO) and other value-added products is an interesting approach for carbon-containing fuel synthesis using renewable and clean energy. The electrochemical reduction of CO2 is one of the promising strategies for the storage of intermittent renewable energy resources. The development of electrocatalysts with high activity and stability is vital in the electrochemical CO2 reduction process. In this study, copper and gold alloyed (CuAu) electrodes with nanodendritic structures were synthesized using a facile electrodeposition method. The CuAu nanodendrites with the atomic ratio of Cu to Au being approximately 1:1 demonstrated excellent catalytic activity for the electrochemical reduction of CO2. Syngas, which is utilized as an intermediate in the production of synthetic natural gas, ammonia, and methanol, was the major product obtained under various applied potentials. Electrochemical impedance spectroscopic (EIS) measurements revealed that the CuAu nanodendrtic catalyst had a much lower charge transfer resistance than Cu and Au electrodeposited catalysts. The CuAu nanodendrite catalyst is an intriguing material with potential applications for syngas production from CO2.

/
| 〈 |
|
〉 |