

硫酸盐功能电解液增强水系钠离子电池NaTi2(PO4)3/C负极材料电化学性能的研究
收稿日期: 2021-01-25
修回日期: 2021-03-01
网络出版日期: 2021-03-20
基金资助
中央高校基本科研业务费专项资金项目(2572019BC15)
Functional Sulfate Electrolytes Enable the Enhanced Cycling Stability of NaTi2(PO4)3/C Anode Material for Aqueous Sodium-Ion Batteries
Received date: 2021-01-25
Revised date: 2021-03-01
Online published: 2021-03-20
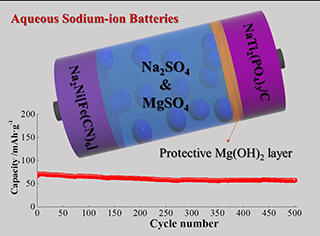
水系钠离子电池具有钠资源丰富、成本低廉、安全可靠、维护简单等特点,在可再生能源规模储存领域具有重要应用前景。NASICON型NaTi2(PO4)3具有可逆容量高、工作电位低、离子传输快等优点,是目前最受关注的水系钠离子电池负极材料。但是,该材料在传统的水系电解液中结构不稳定,循环性能不足。本论文通过调控Na2SO4浓度和引入MgSO4添加剂,构建了一种新型硫酸盐功能电解液(2 mol·L-1 Na2SO4 + 0.3 mol·L-1 MgSO4)。该电解液能够显著增强NaTi2(PO4)3/C材料在充放电循环过程中的结构稳定性,从而提高其电化学可逆性和稳定性。电化学测试表明,NaTi2(PO4)3/C基于该电解液在100 mA·g-1条件下的可逆容量为93.4 mAh·g-1,循环100次后容量保持率高达96.5%;基于该电解液构建的Na2Ni[Fe(CN)6]|NaTi2(PO4)3/C电池可以稳定循环500次以上。本论文结合XRD、XPS等技术讨论分析了该电解液的功能作用机制,其研究结果为设计低成本高性能水系钠离子电池提供了新思路和实验基础。
李姝谨 , 曹志康 , 王文凯 , 张晓菡 , 向兴德 . 硫酸盐功能电解液增强水系钠离子电池NaTi2(PO4)3/C负极材料电化学性能的研究[J]. 电化学, 2021 , 27(6) : 605 -613 . DOI: 10.13208/j.electrochem.210125
Aqueous sodium-ion batteries show promising application in fields of large-scale storage of intermittent renewable energies owing to the earth-abundant sodium resources and incombustible aqueous electrolytes. Primary factors determining whether they can be commercially utilized are low cost and long lifetime. Among current electrode materials, NASICON-type NaTi2(PO4)3 arouses wide interests as an anode material for aqueous sodium-ion batteries as it offers a high specific capacity, fast Na-transport ability and reasonable working potential, however, suffering from insufficient cycling performance caused by severe dissolution of active materials in traditional aqueous electrolytes. In this work, a functional sulfate electrolyte (2 mol·L-1 Na2SO4 + 0.3 mol·L-1 MgSO4) was designed by coupling concentrated Na2SO4 salt and functional MgSO4 additive to enhance the cycling stability of NaTi2(PO4)3/C material. Experimental results from cyclic voltammetry and galvanostatic measurements suggest that the electrolyte can improve electrochemical reversibility and cycling performance of NaTi2(PO4)3/C material relative to traditional electrolyte (1 mol·L-1 Na2SO4). In specific, the material harvested a reversible capacity of 93.4 mAh·g-1 and impressive capacity retention of 96.5% at the specific current of 100 mA·g-1 in the functional sulfate electrolyte, but exhibited a reversible capacity of 88.6 mAh·g-1 and much lower capacity retention of 72.1% in the traditional electrolyte. In order to explore intrinsic causes of the performance improvement, structural properties of the material before and after cycling were comparatively investigated by using X-ray diffraction and X-ray photon spectroscopy. It is found that the material showed excellent structural stability and formation of protective Mg-containing interfacial layer during cycling in the functional sulfate electrolyte. Both the raised electrolyte-salt concentration and functional MgSO4 additive should be responsible for the enhanced structural stability. The high electrolyte-salt concentration could decrease electrochemical activity and widen electrochemical stability window of electrolyte solvents, while the MgSO4 additive could timely capture the hydroxyl group resulting from water-solvent decomposition to prevent the alkalization of aqueous electrolytes and spontaneously form protective Mg(OH)2 interfaces. As a result, the electrolyte could suppress the dissolution of active NaTi2(PO4)3, thus, resulting in the enhanced structural stability and cycling performance. With an aim to further exhibit the feasibility for practical application, full aqueous sodium-ion batteries were assembled by coupling Na2Ni[Fe(CN)6] cathode, functional sulfate electrolyte and NaTi2(PO4)3/C anode. Charge/discharge tests show that the battery could deliver a working voltage of 1.3 V and a reversible capacity of 84.2 mAh·g-1 (calculated as the mass of active anode material) at the current of 100 mA·g-1, achieving a specific energy of about 110 Wh·kg-1. After being continuously charging and discharging for 500 cycles at the current of 500 mA·g-1, it achieved high capacity retention of 80%. The results in this work suggest that designing functional additive-containing sulfate electrolytes is an effective strategy to fabricate low-cost, long-lifetime aqueous sodium-ion batteries.

/
| 〈 |
|
〉 |