

应用镍超微电极的电化学表面增强拉曼光谱技术研究
收稿日期: 2021-01-29
修回日期: 2021-03-18
网络出版日期: 2021-03-20
基金资助
国家自然科学基金项目(21872094);国家自然科学基金项目(21991152);国家自然科学基金项目(21802057)
Electrochemical Surface-Enhanced Raman Spectroscopic Studies on Nickel Ultramicroelectrode
Received date: 2021-01-29
Revised date: 2021-03-18
Online published: 2021-03-20
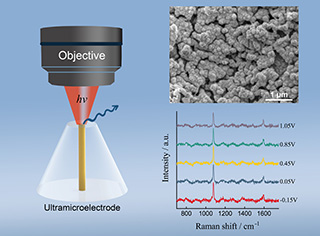
镍(Ni)电极在电化学中应用广泛。原位表征Ni电极表面的吸附物种有益于帮助理解电极反应历程、指导发展高效电催化剂。应用超微电极作为工作电极的电化学表面增强拉曼光谱技术结合了超微电极表面的传质特性和分子水平的高灵敏度表征,是研究Ni电化学的有力手段。本文所述的研究工作通过在金(Au)超微电极表面电吸附具有SERS活性的Au纳米粒子并恒电流沉积金属Ni薄层,制备并表征了具有SERS活性的Ni超微电极。在氢氧化钠溶液中的循环伏安实验和以4-甲基苯硫酚分子作为探针分子的SERS实验结果表明,沉积速率和沉积电量是影响超微电极表面Ni的覆盖度和SERS活性的关键因素。在吸附了直径为55 nm Au纳米粒子的、直径为10 μm Au的超微电极表面,以100 μA·cm-2电流密度电沉积厚度约为5个原子层Ni的条件下,可获得Ni覆盖完好的、具有最强SERS活性的Ni超微电极。
吴丽文 , 王玮 , 黄逸凡 . 应用镍超微电极的电化学表面增强拉曼光谱技术研究[J]. 电化学, 2021 , 27(2) : 208 -215 . DOI: 10.13208/j.electrochem.201245
Nickel (Ni) electrodes are widely used in electrochemical researches. Understanding electrochemical processes on Ni electrodes through in-situ characterization of adsorbed species on their surfaces is helpful for rational optimization and application of Ni electrochemistry. Microelectrochemical surface-enhanced Raman spectroscopy (μEC-SERS) combines the mass transfer feature of ultramicroelectrode with high-sensitivity characterizations of molecular structures, which is a powerful method for studying Ni electrochemistry on polarization and non-equilibrium conditions. The key point of performing μEC-SERS is to make a SERS-active Ni ultramicroelectrode.
Here, we demonstrate a method of preparing a SERS-active Ni ultramicroelectrode through electrochemical deposition of several atomic layers of metallic Ni onto a SERS-active gold (Au) ultramicroelectrode. Firstly, a SERS-active Au ultramicroelectrode was made through electrochemical adsorption of Au nanoparticles. A smooth polycrystalline Au ultramicroelectrode with a diameter of 10 μm was made by sealing a Au wire into a glassy tube. The Au nanoparticles of 55 nm in diameter were adsorbed from Au sol onto the Au ultramicroelectrode under an electrochemical polarization at 1.8 V. The scanning electron microscopic (SEM) images showed that Au nanoparticles aggregated on surface.
On the prepared Au ultramicroelectrode adsorbed by Au nanoparticles, a thin and compact Ni layer was deposited by using galvanostatic method in 5 mmol·L-1 Ni(NO3)2 electrolyte. The thickness of Ni layer was controlled via the charge. The voltammograms of the prepared SERS-active Ni ultramicroelectrode in 0.1 mol·L-1 NaOH showed the characters of polycrystalline Ni electrode. Since the SERS activity decreased as a result of the increase in the thickness of Ni layer, the SERS measurements of 4-methylthiophenol in air were carried out for evaluating SERS activity. The comparisons in the intensity of the band at 1077 cm-1 from the 4-methylthiophenol adsorbed on the ultramicroelectrode made by using 10 μA·cm-2, 50 μA·cm-2, 100 μA·cm-2, 500 μA·cm-2 and 1000 μA·cm-2 indicated that the rate and charge of deposition are key in determining the coverage status of Ni layer and the SERS activity. An optimized SERS activity on a compact Ni was obtained by electrodepositing 5 atomic layers of Ni at a current density of 100 μA·cm-2.
To demonstrate the application of Ni ultramicroelectrode in the in-situ μEC-SERS measurement, the molecule of 4-methylthiophenol, employed as a probe, was adsorbed onto the prepared Ni ultramicroelectrode through spontaneous adsorption in the methanol solution of 4-methylthiophenol. The obtained SERS spectra showed characteristic bands of 4-methylthiophenol. In addition, stark effect of the bands was observed, indicating the successful application of Ni ultramicroelectrode in the in-situ μEC-SERS measurement.
The preparation methodology of SERS-active ultramicroelectrode enables the in-situ μEC-SERS measurement on Ni under electrochemical polarization or non-equilibrium reaction conditions, which exhibits a good potential application in studying Ni electrochemistry.

| [1] | Ni W Y, Krammer A, Hsu C S, Chen H M, Schuler A, Hu X L. Ni3N as an active hydrogen oxidation reaction catalyst in alkaline medium[J]. Angew. Chem. In. Ed., 2019,58(22):7445-7449. |
| [2] | Lu X F, Yu L, Lou X W. Highly crystalline Ni-doped FeP/carbon hollow nanorods as all-pH efficient and durable hydrogen evolving electrocatalysts[J]. Sci. Adv., 2019, 5(2): eaav6009. |
| [3] | Wang Q, Huang X, Zhao Z L, Wang M Y, Xiang B, Li J, Feng Z X, Xu H, Gu M. Ultrahigh-loading of Ir single atoms on NiO matrix to dramatically enhance oxygen evolution reaction[J]. J. Am. Chem. Soc., 2020,142(16):7425-7433. |
| [4] | Stamenkovic V R, Fowler B, Mun B S, Wang G F, Ross P N, Lucas C A, Markovic N M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability[J]. Science, 2007,315(5811):493-497. |
| [5] | Yang H B, Hung S F, Liu S, Yuan K D, Miao S, Zhang L P, Huang X, Wang H Y, Cai W Z, Chen R, Gao J J, Yang X F, Chen W, Huang Y Q, Chen H M, Li C M, Zhang T, Liu B. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction[J]. Nat. Energy, 2018,3(2):140-147. |
| [6] | Wang H L, Casalongue H S, Liang Y Y, Dai H J. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials[J]. J. Am. Chem. Soc., 2010,132(21):7472-7477. |
| [7] | Sun H C, Qin D, Huang S Q, Guo X Z, Li D M, Luo Y H, Meng Q B. Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique[J]. Energy Environ. Sci., 2011,4(8):2630-2637. |
| [8] | Bard A J, Faulkner L R. Electrochemical methods: fundamentals and applications[M]. New York: John Wiley & Sons, Inc., 2001: 168-176. |
| [9] | Fleischmann M, Hendra P J, McQuillan A J. Raman spectra of pyridine adsorbed at a silver electrode[J]. Chem. Phys. Lett., 1974,26(2):163-166. |
| [10] | Albrecht M G, Creighton J A. Anomalously intense Raman spectra of pyridine at a silver electrode[J]. J. Am. Chem. Soc., 1977,99(15):5215-5217. |
| [11] | Jeanmaire D L, Van Duyne R P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode[J]. J. Electroanal. Chem., 1977,84(1):1-20. |
| [12] | Wu D Y, Li J F, Ren B, Tian Z Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures[J]. Chem. Soc. Rev., 2008,37(5):1025-1041. |
| [13] | Kostecki R, McLarnon F. Electrochemical and in situ Raman spectroscopic characterization of nickel hydroxide electrodes: I. Pure nickel hydroxide[J]. J. Electrochem. Soc., 1997,144:485-493. |
| [14] | Diaz-Morales O, Ferrus-Suspedra D, Koper M T M. The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation[J]. Chem. Sci., 2016,7(4):2639-2645. |
| [15] | Louie M W, Bell A T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen[J]. J. Am. Chem. Soc., 2013,135(33):12329-12337. |
| [16] | Wang Y H, Wang X T, Ze H, Zhang X G, Radjenovic P M, Zhang Y J, Dong J C, Tian Z Q, Li J F. Spectroscopic verification of adsorbed hydroxyl intermediate in the bifunctional mechanism of hydrogen oxidation reaction[J]. Angew. Chem. Int. Ed., 2021,60(11):5708-5711. |
| [17] | Kim B J, Lee D J, Kim Y R, Lim S Y, Bae J H, Kim K B, Chung T D. Gold microshell tip for in situ electrochemical Raman spectroscopy[J]. Adv. Mater., 2012,24(3):421-424. |
| [18] | Wang W, Huang Y F, Liu D Y, Wang F F, Tian Z Q, Zhan D P. Electrochemically roughened gold microelectrode for surface-enhanced Raman spectroscopy[J]. J. Ele-ctroanal. Chem., 2016,779:126-130. |
| [19] | Huang Y F, Wang W, Guo H Y, Zhan C, Duan S, Zhan D P, Wu D Y, Ren B, Tian Z Q. Microphotoelectrochemical surface-enhanced Raman spectroscopy: toward bridging hot-electron transfer with a molecular reaction[J]. J. Am. Chem. Soc., 2020,142(18):8483-8489. |
| [20] | Liu N Y, Wu L W, Huang Y F. In-situ electrochemical Raman spectroscopy on ultramicroelectrodes[J]. Sci. Sin. Chim., 2021,51(3):256-263. |
| [21] | Ren B, Huang Q J, Cai W B, Mao B W, Liu F M, Tian Z Q. Surface Raman spectra of pyridine and hydrogen on bare platinum and nickel electrodes[J]. J. Electroanal. Chem., 1996,415(1-2):175-178. |
| [22] | Huang Q J, Yao J L, Mao B W, Gu R A, Tian Z Q. Surface Raman spectroscopic studies of pyrazine adsorbed onto nickel electrodes[J]. Chem. Phys. Lett., 1997,271(1-3):101-106. |
| [23] | Tian Z Q, Ren B, Wu D Y. Surface-enhanced Raman scattering: from noble to transition metals and from rough surfaces to ordered nanostructures[J]. J. Phys. Chem. B, 2002,106(37):9463-9483. |
| [24] | Gao J S(高劲松), Ren B(任斌), Huang Q J(黄群健), Tian Z Q(田中群). Surface Raman spectra obtained from various electrodeposited transidon metals[J]. J. Electro-chem. (电化学) 1996,2(3):258-261. |
| [25] | Xia Y Y, Wu Y W, Wu L W, Wang T Y Y, Hang T, Huang Y F, Li M. Two-step electrodeposited 3D Ni nanocone supported Au nanoball arrays as SERS substrate[J]. J. Electrochem. Soc., 2020,167(14):142502. |
| [26] | Fleischmann M, Tian Z Q, Li L J. Raman spectroscopy of adsorbates on thin film electrodes deposited on silver substrates[J]. J. Electroanal. Chem., 1987,217(2):397-410. |
| [27] | Fleischmann M, Tian Z Q. The induction of SERS on smooth Ag by the deposition of Ni and Co[J]. J. Electro-anal. Chem., 1987,217(2):411-416. |
| [28] | Bao F, Li J F, Ren B, Yao J L, Gu R A, Tian Z Q. Synjournal and characterization of Au@Co and Au@Ni core-shell nanoparticles and their applications in surface-enhanced Raman spectroscopy[J]. J. Phys. Chem. C, 2008,112(2):345-350. |
| [29] | Moskovits M. Surface-enhanced spectroscopy[J]. Rev. Mod. Phys., 1985,57(3):783-826. |
| [30] | Willets K A, Van Duyne R P. Localized surface plasmon resonance spectroscopy and sensing[M]. Annual Review of Physical Chemistry, 2007,58:267-297. |
| [31] | Hayazawa N, Inouye Y, Sekkat Z, Kawata S. Metallized tip amplification of near-field Raman scattering[J]. Opt. Commun., 2000,183(1-4):333-336. |
| [32] | St?ckle R M, Suh Y D, Deckert V, Zenobi R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy[J]. Chem. Phys. Lett., 2000,318(1-3):131-136. |
| [33] | Pettinger B, Ren B, Picardi G, Schuster R, Ertl G. Nano-scale probing of adsorbed species by tip-enhanced Raman spectroscopy[J]. Phys. Rev. Lett., 2004,92(9):096101. |
| [34] | Tian Z Q, Ren B, Li J F, Yang Z L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy[J]. Chem. Commun., 2007: 3514-3534. |
| [35] | Li J F, Zhang Y J, Ding S Y, Panneerselvam R, Tian Z Q. Core-shell nanoparticle-enhanced Raman spectroscopy[J]. Chem. Rev., 2017,117(7):5002-5069. |
| [36] | Montelongo Y, Sikdar D, Ma Y, McIntosh A J S, Velleman L, Kucernak A R, Edel J B, Kornyshev A A. Electrotunable nanoplasmonic liquid mirror[J]. Nat. Mater., 2017,16(11):1127-1135. |
| [37] | Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions[J]. Nature Phys. Sci., 1973,241(105):20-22. |
| [38] | Shao Y, Mirkin M V, Fish G, Kokotov S, Palanker D, Lewis A. Nanometer-sized electrochemical sensors[J]. Anal. Chem., 1997,69(8):1627-1634. |
| [39] | Sun P, Mirkin M V. Kinetics of electron-transfer reactions at nanoelectrodes[J]. Anal. Chem., 2006,78(18):6526-6534. |
| [40] | Zhan D, Velmurugan J, Mirkin M V. Adsorption/desorption of hydrogen on Pt nanoelectrodes: evidence of surface diffusion and spillover[J]. J. Am. Chem. Soc., 2009,131(41):14756-14760. |
| [41] | Ma Y, Sikdar D, Fedosyuk A, Ma Y, Sikdar D, Fedosyuk A, Velleman L, Klemme D J, Oh S H, Kucernak ARJ, Kornyshev A A, Edel J B. Electrotunable nanoplasmonics for amplified surface enhanced Raman spectroscopy[J]. ACS Nano, 2020,14(1):328-336. |
| [42] | Nie S, Emory S R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering[J]. Science, 1997,275(5303):1102-1106. |
| [43] | Kneipp K, Wang Y, Kneipp H, Perelman, L T, Itzkan I, Dasari R, Feld M S. Single molecule detection using surface-enhanced Raman scattering (SERS)[J]. Physical Review Letters, 1997,78(9):1667-1670. |
| [44] | Xu H X, Aizpurua J, K?ll M, Apell P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering[J]. Phys. Rev. E, 2000,62(3):4318-4324. |
/
| 〈 |
|
〉 |