

酸性和碱性溶液中金属氮碳材料氧还原和氢析出反应的理论研究
收稿日期: 2021-02-02
修回日期: 2021-03-10
网络出版日期: 2021-03-20
Theoretical Studies of Metal-N-C for Oxygen Reduction and Hydrogen Evolution Reactions in Acid and Alkaline Solutions
Received date: 2021-02-02
Revised date: 2021-03-10
Online published: 2021-03-20
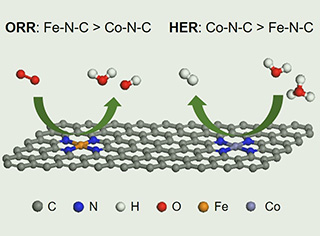
单原子催化剂(SAC)由于其低成本和在各种电催化反应中潜在的高催化活性而被认为是铂族金属的有前景的替代材料,但仍然缺乏对不同金属氮碳材料催化剂之间活性差异的原子机理的理解。在此,通过实验和理论研究相结合,研究了非贵金属氮碳材料(Me-N-C,Me = Fe和Co)作为模型催化剂,以探索在普遍的pH值下氧还原反应(ORR)和氢析出反应(HER)的催化活性以及相对应的反应机理。原子理论模拟表明,Fe-N-C具有比Co-N-C高的ORR活性,这是因为其速率决定步骤的反应势垒较低,而HER的活性趋势却相反。我们的模拟结果与实验观察结果一致。
秦雪苹 , 朱尚乾 , 张露露 , 孙书会 , 邵敏华 . 酸性和碱性溶液中金属氮碳材料氧还原和氢析出反应的理论研究[J]. 电化学, 2021 , 27(2) : 185 -194 . DOI: 10.13208/j.electrochem.201248
Single atom catalysts (SAC) have been regarded as the promising alternatives to platinum group metals due to their low costs and potentially high catalytic activities in various electrocatalytic reactions. The atomic mechanism understanding of activity discrepancy among different metal and nitrogen co-doped carbon-based catalysts is still lacking. Here, non-precious metal and nitrogen co-doped carbons (Me-N-C, Me = Fe and Co) as the model catalysts are investigated by combining experimental and theoretical studies to explore the catalytic activities and corresponding reaction mechanisms toward oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) at universal pHs. Atomic theoretical simulations suggest that Fe-N-C has higher ORR activity than Co-N-C due to its lower reaction barrier of the rate-determining step, while the activity trend is reversed for HER. Our simulation results are consistent with experimental observations.

/
| 〈 |
|
〉 |