

氮掺杂碳原位锚定铜纳米颗粒用于高效氧还原反应催化剂
#两位作者对此文章贡献相同。
收稿日期: 2020-07-24
修回日期: 2021-02-18
网络出版日期: 2021-02-22
基金资助
国家自然科学基金项目(21865025);国家自然科学基金项目(51962032)
Copper Nanoparticles In-Situ Anchored on Nitrogen-Doped Carbon for High-Efficiency Oxygen Reduction Reaction Electrocatalyst
Received date: 2020-07-24
Revised date: 2021-02-18
Online published: 2021-02-22
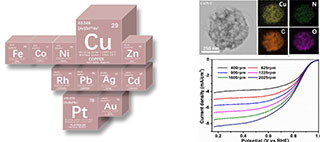
与贵金属铂基电化学氧还原反应(ORR)催化剂相比,廉价的非贵金属催化剂引起了广泛的关注。本文以壳聚糖作为一种富含氮和碳元素的生物质资源,利用碳浴法成功制备了氮掺杂碳原位负载铜纳米颗粒(Cu/N-C)催化剂。纯壳聚糖碳化得到的样品N-C的比表面积为67.5 m2·g-1、平均孔径0.14 nm、平均孔体积8.00 m2·g-1,与之相比,Cu/N-C比表面积可达607.3 m2·g-1、平均孔径为2.5 nm、平均孔体积为0.40 cm3·g-1。通过密度泛函理论(DFT)进行计算表明,Cu(111)/N-C的自由能值低于N-C,更有利于氧还原催化进行。在0.1 mol·L-1 KOH的介质中,Cu/N-C不仅表现出优异的起始和半波电势(分别为0.96 V和0.84 V),而且还表现出了优异的抗甲醇性能和稳定性,并且Cu元素掺杂量达到1.67wt.%。
袁会芳 , 张越 , 翟兴吾 , 胡立兵 , 葛桂贤 , 王刚 , 于锋 , 代斌 . 氮掺杂碳原位锚定铜纳米颗粒用于高效氧还原反应催化剂[J]. 电化学, 2021 , 27(6) : 671 -680 . DOI: 10.13208/j.electrochem.200724
Compared with noble metal platinum (Pt)-based catalysts, inexpensive non-noble metal electrocatalysts have attracted extensive attention for oxygen reduction reaction (ORR). Herein, chitosan as a kind of biomass resource rich in nitrogen and carbon was used to prepare nitrogen-doped carbon (N-C) and N-C in-situ anchored by copper nanoparticles (Cu/N-C). The as-obtained N-C and Cu/N-C nanoparticles were successfully used as non-noble eletrocatalysts tested for ORR. Compared with the N-C, the Cu/N-C showed the high surface area of 607.3 m 2·g-1 with the mean pore size of 2.5 nm and the pore volume of 0.40 cm3·g-1. The most positive Gibbs free energy change was the rate determining step for ORR process with the 4e mechanism, where the value of the Cu(111)/N-C(-0.39 eV) was lower than that of the N-C(-0.26 eV). The Cu/N-C exhibited superior onset and half-wave potentials (0.96 V and 0.84 V, respectively) in alkaline media(0.1 mol·L-1 KOH), all of which are much better than those measured for N-C and commercial Pt/C. Furthermore, the Cu/N-C showed superior methanol crossover avoidance and oxygen reduction stability.

/
| 〈 |
|
〉 |