

宏观均相多孔电极电化学阻抗谱基础
收稿日期: 2020-11-26
修回日期: 2020-12-25
网络出版日期: 2021-02-09
基金资助
国家自然科学基金项目(22078190)
Fundamentals of Electrochemical Impedance Spectroscopy for Macrohomogeneous Porous Electrodes
Received date: 2020-11-26
Revised date: 2020-12-25
Online published: 2021-02-09
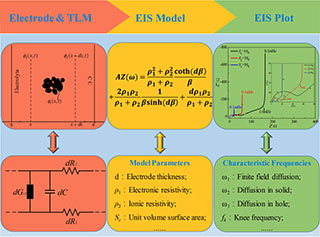
电化学阻抗谱可用于诊断多孔电极内电荷转移反应,即界面电荷集聚和电荷传导,以及反应物质输运。本文采用复相量方法,在同态假设条件下,重新推演多孔电极阻抗谱模型,厘清传统多孔电极阻抗谱模型中的模糊性表述。(1) 定义多孔电极表征输入参数,包括电极基体电子电导率σ1 、电解质离子电导率σ2、界面电荷传递电导率gct、单位面积界面电容C、固相扩散系数D、速度常数k、电极厚度d、特征孔深Lp 和单位体积表面积Sc;(2) 解析阻抗谱特征输出参数,包括场扩散常数K,特征频率ω0、ω1、ω2、ω3和 ωmax,它们分别相关于界面传导反应、有限场扩散、氧化还原反应、孔内扩散和最小特征孔尺寸,以及分别对应于从传导到扩散和从扩散到饱和的转折频率fk1 和fk2;(3) 当参数X和Z同时变化时(X = σ1和Z = d,Sc,Lp,C,gct,D,k),通过阻抗谱特征参数的演变规律,分析了电荷转移反应中X和Ζ参数耦合竞争;(4)为深入分析电荷转移反应中参数X和Z的耦合竞争,引入了分叉频率fXZ和fZX 。fXZ和fZX所处位置可以用于表征参数X和Z影响电荷转移反应的深度和广度。当分叉频率fXZ和fZX不存在时,表明电荷转移反应中参数X和Z在全频率范围内存在耦合竞争。总之,借助于特征频率和分叉频率,本文一方面研究了动力学参数和微观结构参数对多孔电极中电荷转移反应的影响,另一方面分析谱图的变化及其背后的阻抗谱特征演化规律。本文研究结果可为阻抗谱的系统仿真和辨识提供理论基础,可为多孔电极内电荷转移反应的竞争分析提供技术支撑,还可为电化学储能系统的优化设计提供诊断工具。
李响 , 黄秋安 , 李伟恒 , 白玉轩 , 王佳 , 刘杨 , 赵玉峰 , 王娟 , 张久俊 . 宏观均相多孔电极电化学阻抗谱基础[J]. 电化学, 2021 , 27(5) : 467 -497 . DOI: 10.13208/j.electrochem.201126
Electrochemical impedance spectroscopy (EIS) can be used to diagnose charge transfer reactions and mass transport in porous electrodes. The charge transfer reactions include interfacial charge accumulation and charge conduction as well as electrochemical reaction. In this paper, the complex phasor method is developed under the macrohomogeneous assumption to build an impedance model of porous electrodes for clarifying several vague expressions in the traditional approaches. The following researches are carried out: (1) Identifying characteristic parameters for the porous electrodes, including electrode electronic conductivity σ1, electrolyte ionic conductivity σ2, interface charge transfer conductivity gct, unit area interface capacitance C, solid phase diffusion coefficient D, rate constant k, electrode thickness d, characteristic hole depth Lp and unit volume surface area Sc ; (2) elucidating characteristic output parameters for the impedance spectroscopic response, including field diffusion constant K, characteristic frequencies ω0, ω1, ω2, ω3, and ωmax for interface conduction reaction, finite field diffusion, redox reaction, pore diffusion and minimum characteristic pore size, respectively. In addition, the transition frequencies fk1 and fk2 from conduction to diffusion area and from diffusion to saturation area are also defined and studied respectively; (3) defining the parameters X and Z, herein, X = σ1,Z = d、Sc, Lp , C, gct , D, k,which are responsible for the evolution trend of the characteristic parameters for impedance spectroscopic response, the competition effects of X and Z parameters coupled in charge transfer reaction are analyzed; (4) Further analyzing the competition effects of X and Z parameters coupled in the charge transfer reaction, the diverging frequencies fXZ and fXZ are phenomenologically defined. The locations of fXZ and fXZ can indicate the depth and breadth of the charge transfer reaction affected by the parameters X and Z. The non-existence of fXZ and fXZ indicates that the parameter X or Z can affect the charge transfer reaction over the whole frequency range. With the help of characteristic frequency and diverging frequency, the effects of electrode kinetic and microstructure parameters on the charge transfer reaction in porous electrodes are studied; on the other hand, the shape change and trend evolution of the impedance responses for porous electrodes are analyzed. The research results in this paper should be able to provide theoretical basis for system simulation and system identification of impedance spectroscopy, technical support for competitive analysis of charge transfer reaction in porous electrodes, and diagnostic tool for optimal design of electrochemical energy storage system.

/
| 〈 |
|
〉 |