

高导电性和催化活性的Janus-TiNbCO2析氢反应催化材料
收稿日期: 2020-11-09
修回日期: 2020-12-22
网络出版日期: 2021-02-09
基金资助
国家自然科学基金项目(12105236)
Janus-TiNbCO2 for Hydrogen Evolution Reaction with High Conductivity and Catalytic Activity
Received date: 2020-11-09
Revised date: 2020-12-22
Online published: 2021-02-09
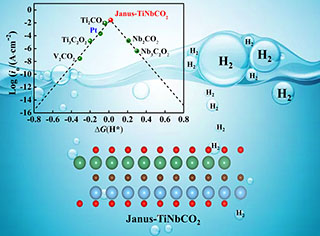
探寻具有高导电性和高催化活性的析氢反应(HER)催化材料一直是可持续能源发展研究中的热点。Ti2C具有表面活性位点多和优良的力学稳定性、导电性等,已成为潜在的制氢催化剂。然而,终端O修饰Ti2C表面,会降低该材料的导电性,进而限制了电子在价带与导带间的输运。本研究通过Nb掺杂,构建双电层Janus-TiNbCO2,并借助VASP软件研究了Janus-TiNbCO2的能带结构、HER性能和HER反应路径过渡态。结果表明,Janus-TiNb-CO2为导体材料,其在应力、氧空位缺陷和H*覆盖度的影响下,均表现出极优异的催化活性,计算获得的最优ΔGH*值为0.02 eV。H*在Janus-TiNbCO2上可能以Heyrovsky路径进行反应,该路径的迁移能势垒为0.23 eV。Janus-TiNbCO2是一种具有HER应用前景的催化材料。
关键词: MXene; Janus-TiNbCO2; 能带; 析氢反应
徐黎黎 , 任冬燕 , 赵骁锋 , 易勇 . 高导电性和催化活性的Janus-TiNbCO2析氢反应催化材料[J]. 电化学, 2021 , 27(5) : 570 -578 . DOI: 10.13208/j.electrochem.201109
Exploring the potential hydrogen evolution reaction (HER) catalysts with the high activity and high conductivity has always been a hot spot in the research of renewable energy development. Ti2C, as one of the 2D-MXene, has excellent properties relating to many active sites, mechanical stability, conductivity, etc., and has become a potential HER catalyst. However, the modification of the surface of Ti2C by terminal O will reduce the conductivity, thereby limiting the transport of electrons between the valence band and the conduction band. In this study, an electric double layer Janus-TiNbCO2 was constructed by Nb doping. The band property, HER activity and HER reaction path of Janus-TiNbCO2 are studied by the first-principles calculations. The results show that Nb doping increases the distance between Ti and O atoms, which increases the lattice parameters of Janus-TiNbCO2 comparing with that of Ti2CO2 structure. The Janus-TiNbCO2 structure is stable by calculating the thermodynamic stability at 500 K using AIMD method. The band gap of Ti2CO2 is approximate 0.9 eV. After Nb doping, the orbital hybridization between Nd-3d and O-2p affects the electronic rearrangement of Ti-3d, leading that Janus-TiNbCO2 has the metal band structure. In Janus-TiNbCO2, both Ti and Nb surfaces adsorb H* by O site, where the ΔGH*(@Ti) = -0.55 eV,ΔGH*(@Nb) = 0.02 eV, showing Ti and Nb surfaces have different catalytic activities. Comparing with graphenes, e.g., h-B2O, Pt, and g-C3N4, Janus-TiNbCO2 has better catalytic activity. The charge distribution of Janus-TiNbCO2 near the Fermi level was analyzed by HSE-06 function. The result reveals that O atoms on the Ti surface exhibit charge unsaturation at the Fermi level, while those on Nb surface strong saturation. Moreover, the effects of H* coverage and strains(+2% ~ +4%) on the catalyst activity of Janus-TiNbCO2 are studied. When the H* coverage is low, the optimal ΔGH* of Nb surface is approximate 0.02 eV, while Ti surface has an excellent catalytic activity at high H* coverages (θ = 7/9, ΔGH* = -0.06 eV). Under the strain action, the H* coverage on surface is not affected. However, strains will reduce the HER activity of Nb surface, and increase the HER activity of Ti surface. Furthermore, oxygen defect is a stable point defect in Janus-TiNbCO2 . Oxygen defect will increase the HER activity of Ti surface and decrease the HER activity of Nb surface. Comparing to the Tafel pathway, the Heyrovsky is a more suitable pathway for the HER, in which the migration barrier of Heyrovsky is 0.23 eV for H* on Nb surface. Janus-TiNbCO2 can be used as a potential HER catalyst.

Key words: MXene; Janus-TiNbCO2; band; hydrogen evolution reaction
| [1] | Bai X W, Ling C Y, Shi L, Ouyang Y X, Li Q, Wang J L. Insight into the catalytic activity of MXenes for hydrogen evolution reaction[J]. Sci. Bull., 2018, 63(21): 1397-1403. |
| [2] | Ling C Y, Shi L, Ouyang Y X, Wang J L. Searching for highly active catalysts for hydrogen evolution reaction based on O-terminated MXenes through a simple descriptor[J]. Chem. Mater., 2016, 28(24): 9026-9032. |
| [3] | Zheng J N, Sun X, Qiu C L, Yan Y L, Yao Z H, Deng S W, Zhong X, Zhuang G L, Wei Z Z, Wang J G. High-throughput screening of hydrogen evolution reaction catalysts in MXene materials[J]. J. Phys. Chem. C, 2020, 124(25): 13695-13705. |
| [4] | Li P K, Zhu J G, Handoko A D, Zhang R F, Wang H T, Legut D, Wen X D, Fu Z H, She Z W, Zhang Q F. High-throughput theoretical optimization of the hydrogen evolution reaction on MXenes by transition metal modification[J]. J. Mater. Chem. A, 2018, 6(10): 4271-4278. |
| [5] | Zhang S Z(张绍政), Liu J(刘佳), Xie Y(谢艳), Lu Y J(陆银稷), Li L(李林), Lü L(吕亮), Yang J H(杨建辉), Wei S H(韦世豪). First-principles study of hydrogen evolution activity for two-dimensional M2XO2-2xOH2x(M = Ti, V; X = C, N)[J]. Acta Phy. - Chim. Sin.(物理化学学报), 2017, 33: 2022-2028. |
| [6] | Meshkian R, Näslund L Å, Hallim J, Lu J, Barsoum M W, Rosen J. Synjournal of two-dimensional molybdenum carbide, MO2C, from the gallium based atomic laminate MO2Ga2C[J]. Scripta Mater., 2015, 108: 147-150. |
| [7] | Ding B, Ong W J, Jiang J, Ding B, Ong W J, Jiang J Z, Chen X Z, Li N. Uncovering the electrochemical mechanisms for hydrogen evolution reaction of heteroatom doped M2C MXene (M = Ti, Mo)[J]. Appl. Surf. Sci., 2020, 500: 143987. |
| [8] | Zhang Y J, Wang L, Zhang N N, Zhou Z J. Adsorptive environmental applications of MXene nanomaterials: a review[J]. RSC Adv., 2018, 8(36): 19895-19905. |
| [9] | Taheri-Qazvini N, Snyder S A, Jang M, Heo J, Yoon Y. Applications of MXene-based membranes in water purification: a review[J]. Chemosphere, 2020, 254: 126821. |
| [10] | Hu T, Yang J X, Wang X H. Carbon vacancies in Ti2CT2 MXenes: defects or a new opportunity?[J]. Phys. Chem. Chem. Phys., 2017, 19(47): 31773-31780. |
| [11] | Yang X Y, Luo W, Ahuja R. Fluoride ion batteries: designing flexible M2CH2 (M = Ti or V) MXenes as high-capacity cathode materials[J]. Nano Energy, 2020, 74: 104911. |
| [12] | Huang B, Zhou N G, Chen X Z, Ong W J, Li N. Insights into the electrocatalytic hydrogen evolution reaction mechanism on two-dimensional transition-metal carbonitrides (MXene)[J]. Chem. - Eur. J., 2018, 24(69): 18479-18486. |
| [13] | Zhou X(周雪), Wang H(王虹), Yin Z(尹振), Zhang Y J(张玉军), Li J X(李建新). Preparations of nano-MnOx/Ti electrocatalytic membrane electrode for catalytic oxidation of cyclohexane using intermittent electrodeposition[J]. J. Electrochem.(电化学), 2020, 26(3): 397-405. |
| [14] | Wang S, Chen L, Wu Y, Zhang Q J. Surface modifications of Ti2CO2 for obtaining high hydrogen evolution reaction activity and conductivity: A computational approach[J]. ChemPhysChem, 2018, 19(24): 3380-3387. |
| [15] | Li L, Wang X Y, Guo H R, Yao G, Yu H B, Tian Z Q, Li B H, Chen L. Theoretical screening of single transition metal atoms embedded in MXene defects as superior electrocatalyst of nitrogen reduction reaction[J]. Small Methods, 2019, 3(11): 1900337. |
| [16] | Zhu J, Ha E N, Zhao G L, Zhou Y, Huang D S, Yue G Z, Hu L S, Sun N, Wang Y, Lee L Y S, Xu C, Wong K Y, Astruc D, Zhao P X. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption[J]. Coordin. Chem. Rev., 2017, 352: 306-327. |
| [17] | Henkelman G, Uberuaga B P, Jonsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J. Chem. Phys., 2000, 113(22): 9901-9904. |
| [18] | Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comp. Mater. Sci., 1996, 6(1): 15-50. |
| [19] | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Phys. Rev. Lett., 1996, 77(18): 3865-3868. |
| [20] | Perdew J P, Emzerhof M, Burke K. Rationale for mixing exact exchange with density functional approximations[J]. J. Chem. Phys., 1996, 1996(105): 9982-9985. |
| [21] | Heyd J, Scuseria G E, Ernzerhof M. Hybrid functionals based on a screened coulomb potential[J]. J. Chem. Phys., 2003, 118(18): 8207-8215. |
| [22] | Gao G, O’Mullane A P, Du A. 2D MXenes: a new family of promising catalysts for the hydrogen evolution reaction[J]. ACS Catal., 2016, 7(1): 494-500. |
| [23] | Zhao X F, Yang X Y, Singh D, Panda P K, Luo W, Li Y X, Ahuja R. Strain-engineered metal-free h-B2O monolayer as a mechanocatalyst for photocatalysis and improved hydrogen evolution reaction[J]. J. Phys. Chem. C, 2020, 124(14): 7884-7892. |
| [24] | Mishra A, Satsangi S, Rajan A C, Mizuseki H, Lee K R, Singh A K. Accelerated data-driven accurate positioning of the band edges of MXenes[J]. J. Phys. Chem. Lett., 2019, 10(4): 780-785. |
| [25] | Zhao X F, Panda P K, Singh D, Yang X Y, Mishra Y K, Ahuja R. 2D g-C3N4 monolayer for amino acids sequencing[J]. Appl. Surf. Sci., 2020, 528: 146609. |
| [26] | Sinthika S, Waghmare U V, Thapa R. Structural and electronic descriptors of catalytic activity of graphene-based materials: first-principles theoretical analysis[J]. Small, 2018, 14(10): 1703609. |
/
| 〈 |
|
〉 |