

锂金属电池用三维半互穿网络聚合物电解质的制备
收稿日期: 2020-09-27
修回日期: 2020-10-10
网络出版日期: 2020-11-10
基金资助
国家重点研发计划项目(2018YFB1502903);国家自然科学基金项目(21603197)
Preparation of 3D Semi-Interpenetrated Polymer Networks Polymer Electrolyte for Lithium Metal Battery
Received date: 2020-09-27
Revised date: 2020-10-10
Online published: 2020-11-10
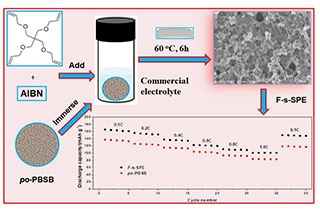
锂金属电池作为下一代高比能量电池技术受到人们越来越广泛的关注。然而由锂枝晶生长引发的安全问题是锂金属电池商业化面临的最大挑战之一。具有高锂离子迁移数和离子电导率的聚合物电解质是抑制锂枝晶生长的重要策略之一。本文将季戊四醇四丙烯酸酯和自由基引发剂AIBN添加至商业化电解液中,采用具有单离子传导功能的多孔聚合物电解质为锂金属电池的电解质隔膜,通过在电池内部发生热诱导原位聚合制备三维半互穿网络单离子传导聚合物电解质,达到提高电解质隔膜离子电导率和机械拉伸性能,以及有效抑制锂枝晶生长的目的。通过该策略的实施,成功获得了室温离子电导率0.53 mS·cm-1和锂离子迁移数0.65的良好结果。应用于锂金属电池,证明该电解质能够有效抑制锂枝晶的生长和倍率性能的提高,为锂金属电池的开发提供了良好的解决路径。
张运丰 , 王佳颖 , 李晓洁 , 赵诗宇 , 何阳 , 霍士康 , 王雅莹 , 谭畅 . 锂金属电池用三维半互穿网络聚合物电解质的制备[J]. 电化学, 2021 , 27(4) : 413 -422 . DOI: 10.13208/j.electrochem.200915
As the next generation high-energy batteries, lithium metal battery has attracted more and more attention due to its highest specific capacity (3860 mA·h·g-1) and the lowest anode potential (-3.04 V versus the standard hydrogen electrode, SHE). However, the safety problem caused by lithium dendrite growth is one of the biggest challenges for the commercialization of lithium metal batteries. Single ion conducting polymer electrolytes, which deliver high lithium ion transference number, represent one of the important strategies to inhibit lithium dendrite growth. However, the poor compatibility with electrodes and low ionic conductivity largely limit their practical application. In the present work, the cross-linking pentaerythritol tetraacrylate precursor and AIBN radical initiator was select as an additive in the commercial 1 mol·L-1 LiPF6-EC/PC (v:v = 1:1) electrolyte, and then was added into the high porous single ion conducting polymer electrolyte. The as-prepared single ion conducting polymer electrolyte was used as the polymer electrolyte for assembling lithium metal battery with the LiFePO4 cathode. The three-dimensional semi-interpenetrating network inside the high porous single ion conducting polymer electrolyte was fabricated by thermal-induced in-situ polymerization inside of the battery by putting the battery in an oven at high temperature. The key properties were successfully investigated. The results indicated that the formed three-dimensional semi-interpenetrating network of the single ion conducting polymer electrolyte was great favorable to improve the ionic conductivity and mechanical property of the polymer electrolyte, and subsequently, to effectively inhibit the growth of lithium dendrite. As a result, the ionic conductivity of 0.53 mS·cm-1 at room temperature and lithium ion transference number of 0.65 were successfully obtained through the implementation of this strategy. It is proved that the as-presented electrolyte can effectively inhibit the growth of lithium dendrite and improve the rate performance, which provides a facile solution for the development of lithium metal battery technology.

/
| 〈 |
|
〉 |