

类石墨烯类活性炭材料的简易合成及其在锂硫电池中的应用研究
收稿日期: 2020-06-28
修回日期: 2020-09-01
网络出版日期: 2020-09-21
Facile Synthesis of Nitrogen-Doped Graphene-Like Active Carbon Materials for High Performance Lithium-Sulfur Battery
Received date: 2020-06-28
Revised date: 2020-09-01
Online published: 2020-09-21
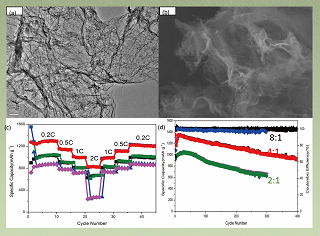
锂硫电池由于具有较高的理论容量被视为一种最具发展潜力的储能装置. 然而,硫的利用率较低及循环寿命短等问题限制着其商业化进程. 本文通过一种简单易行的方法将三聚氰胺(C3H6N6)和L半胱氨酸(C3H7NO2S)碳化,制备出一种氮掺杂类石墨烯活性炭材料(NGC). 该材料的类石墨烯结构能够有效抑制锂硫电池在充放电过程中产生的体积效应,以此提升其循环性能. 不仅如此,材料中含有的含氮官能团还可以促进离子转移,抑制多硫化物的溶解,进而提升硫的利用率. 其中,制备出的NGC-8/PS复合电极用于锂硫电池时在0.2 C的电流密度下初始容量为1164.1 mAh·g-1,在经过400圈的充放电循环之后依然具有909.4 mAh·g-1的比容量,每圈容量衰减仅为0.05%,甚至在2C的电流密度下也能达到820 mAh·g-1的高比容量.
孟全华 , 邓雯雯 , 李长明 . 类石墨烯类活性炭材料的简易合成及其在锂硫电池中的应用研究[J]. 电化学, 2020 , 26(5) : 740 -749 . DOI: 10.13208/j.electrochem.200646
Lithium-sulphur (Li-S) battery is regarded as a promising energy storage device because of its high theoretical capacity. However, the low S utilization and short cycling life limit the commercial applications. In this work, nitrogen-doped graphene-like carbon (NGC) materials were synthesized by simply pyrolyzing and carbonizing the mixture of melamine (C3H6N6) and L-cysteine (C3H7NO2S). The graphene-like structure in NGC effectively buffered the volume change of S during the discharge/charge process and improved the cycling stability. Meanwhile, nitrogen-containing functional groups in NGC facilitated the transportation of ions and suppressed the dissolution of polysulphide (PS), enabling a high utilization of S. As expected, the NGC-8 (the mass ratio of melamine and L-cysteine being 8:1)/PS cathode delivered a high initial discharge capacity of 1164.1 mAh·g-1 at 0.2 C and still retained 909.4 mAh·g-1 capacity after 400 cycles with a slow capacity decay rate of 0.05% per cycle. Even at as high as 2 C, a high-rate capacity of 820 mAh·g-1 could be achieved.

/
| 〈 |
|
〉 |