

多孔电极在电化学体系中的应用
收稿日期: 2020-07-06
修回日期: 2020-09-21
网络出版日期: 2020-10-28
Porous Electrodes in Electrochemical Energy Storage Systems
Received date: 2020-07-06
Revised date: 2020-09-21
Online published: 2020-10-28
吉维肖 , 王功伟 , 王强 , 白力军 , 屈德扬 . 多孔电极在电化学体系中的应用[J]. 电化学, 2020 , 26(5) : 576 -595 . DOI: 10.13208/j.electrochem.200649
Professor C.S. Cha was among the pioneers who have introduced modern electrochemistry to China. Under his leadership, the electrochemical research group in Wuhan University became one of the global powerhouses in fundamental and applied electrochemical researches. During the past many decades, Professor Cha and his colleagues in the university have educated and trained many students who have become part of the backbone of electrochemistry worldwide. In this review, we demonstrate the solid foundation laid by Professor Cha and his colleagues in Wuhan University, and the advancements made in the area of porous electrodes by the authors. All the authors in this paper graduated from Wuhan University at different times. We are fortunate to be standing on the shoulders of a giant.
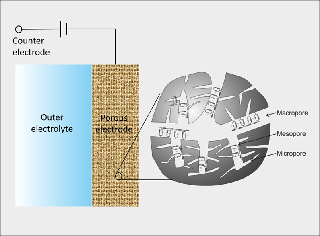
/
| 〈 |
|
〉 |