

光电流谱、光致发光光谱和紫外可见吸收光谱在纳米半导体光电器件研究中的联用
收稿日期: 2020-07-28
修回日期: 2020-08-04
网络出版日期: 2020-08-25
基金资助
国家自然科学基金项目(21533006);国家自然科学基金项目(21273182);国家重点研发计划(2018YFC1602805)
Combined Applications of Photocurrent Spectroscopy, Photoluminescence Spectroscopy and UV-Vis Spectroscopy for Nano-Semiconductor Based Photoelectric Devices
Received date: 2020-07-28
Revised date: 2020-08-04
Online published: 2020-08-25
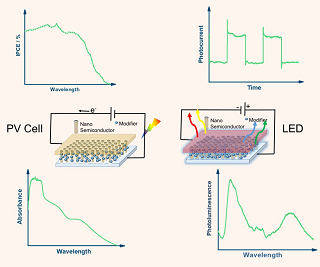
在纳米半导体中由于纳米效应(如量子尺寸效应),其电子结构与块体半导体有所不同。进一步地,当纳米半导体与基底和其他组分结合制成器件后,其性质又受到基底或其他组分的影响,这两点导致了基于纳米半导体的光电器件的性能以及相应表征方法也大不相同。将光电流谱、光致发光光谱和紫外可见吸收光谱三种技术有机地结合起来,可以更好地表征纳米半导体的电子性质和光电性能。本文根据纳米半导体材料与电极的电子性质特点及其测量,结合本课题组前期工作,举例介绍三种谱学方法相结合应用于探究光伏电池和电致发光器件的纳米半导体材料的性能,以及纳米半导体材料表面态的表征。
卞斯达 , 周剑章 , 林仲华 . 光电流谱、光致发光光谱和紫外可见吸收光谱在纳米半导体光电器件研究中的联用[J]. 电化学, 2021 , 27(1) : 45 -55 . DOI: 10.13208/j.electrochem.200728
The electronic structures and properties of nano-semiconductors are quite different from those of bulk semiconductors due to the nano-size effect (such as quantum size effect). Moreover, when the nano-semiconductor materials are deposited onto the substrate to construct a device, their electronic properties are also affected by the substrate or other components, which may lead to different performances of nano-semiconductors based photoelectric devices, and consequently, different corresponding characterization methods are needed. The combination of photocurrent spectroscopy, photoluminescence spectroscopy with UV-Vis absorption spectroscopy can provide a more comprehensive characterization for the electronic properties and photoelectrochemical performances of nano-semiconductors in photoelectric devices. Our research group has long devoted to the studies in the preparations and characterizations of different nano-semiconductors for photoelectric devices. In this review, we firstly introduce the main different features in electronic properties of nano-semiconductors and the corresponding characterization methods, and then describe how to combine the abovementioned three spectroscopic methods to investigate the electronic properties and photoelectrochemical performances of the nano-semiconductors for photovoltaic cells, electroluminescent diodes, and other photocatalytic systems by detailed examples. Photoluminescence spectroscopy is a common method to characterize the surface states of the semiconductors, while photocurrent spectroscopy can provide the supplementary information of surface states. Especially, the photoluminescence efficiency is low for the nonradiactive recombination dominated materials such as indirect-bandgap semiconductors. The results of photocurrent spectroscopy are more useful under these circumstances. Furthermore, the combined characterization in surface states of the nano-semiconductors by photocurrent spectroscopy with photoluminescence spectroscopy is specially discussed. The combined applications of these three spectroscopic methods for the nano-semiconductors in photoeltric devices are not only beneficial to obtain intensive understanding the electric properties and the photo-induced charge transfer mechanism at the interface of the nano-semiconductors, but also useful to guide the preparation of nano-semiconductor materials for photoelectric devices and optimal to the photoelectrochemical performances.

/
| 〈 |
|
〉 |