

Li-SGO掺杂半互穿网络型多孔单离子传导聚合物复合电解质的制备
收稿日期: 2020-04-16
修回日期: 2020-07-01
网络出版日期: 2020-07-01
基金资助
科技部重点研发计划项目(2018YFB1502903);国家自然科学基金项目(21603197)
Preparation and Performance Investigation of Li-SGO doped Semi-IPNs Porous Single Ion Conducting Polymer electrolyte
Received date: 2020-04-16
Revised date: 2020-07-01
Online published: 2020-07-01
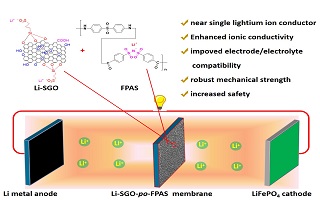
本文成功制备了磺酸锂功能化石墨烯,通过原位聚合方式成功将其添加到单离子传导聚合物电解质中制备出磺酸锂功能化石墨烯改性半互穿网络型多孔单离子传导聚合物复合电解质。与未掺杂磺酸锂功能化石墨烯半互穿网络型多孔单离子传导聚合物电解质相比,该电解质具有更高的孔隙率、吸液率、机械拉伸强度和离子电导率。电化学测试结果表明,掺杂磺酸锂功能化石墨烯后,单离子传导聚合物电解质表现出与电极界面更好的相容性,组装的Li|LiFePO4锂离子电池表现出良好的循环性能和更高的倍率性能。对氧化石墨烯磺酸锂功能化可应用于对单离子传导聚合物电解质的改性,有助于提升单离子传导聚合物电解质的综合性能,获得更高的电池性能。
关键词: 锂离子电池; 单离子传导聚合物电解质; 孔隙率; 离子电导率; 磺化氧化石墨烯
张运丰 , 董佳明 , 谭畅 , 霍士康 , 王佳颖 , 何阳 , 王雅莹 . Li-SGO掺杂半互穿网络型多孔单离子传导聚合物复合电解质的制备[J]. 电化学, 2021 , 27(1) : 108 -117 . DOI: 10.13208/j.electrochem.200409
Herein, the lithiated sulfonated graphene oxide (Li-SGO) was successfully prepared via three steps by sulfonation of graphene oxide with 3-merraptnpropylt rimethnxysilane, oxidation of thiol into sulfonate with hydrogen peroxide and lithiation of sulfonate with aqueous lithium hydroxide. The as-prepared Li-SGO was then introduced into the semi-interpenetrating networks of single ion conducting polymer electrolyte (Li-SGO-FPAS) and poly vinylidenefluoride-hexafluoro propylene (PVDF-HFP) binder by in-situ polymerization to fabricate the porous single ion conducting polymer electrolyte membrane (Li-SGO-po-FPAS) generated from the poor compatibility between aromatic Li-SGO-FPAS and aliphatic PVDF-HFP binder. The key properties such as morphology, porosity, solvent uptake, mechanical strength, flexibility, lithium ion transference number, ionic conductivity and rate-capacity were successfully investigated. In addition, the neat single ion polymer electrolyte membrane without Li-SGO (FPAS) (po-FPAS) was prepared for comparison. The Li-SGO-po-FPAS possessed the high porosity of 55.9% and electrolyte uptake of 139.3wt.%, which are much higher than the values derived from the PP separator. As a result, the enhanced ionic conductivities of 0.23 mS·cm-1 and 1.84 mS·cm-1 were obtained at room temperature and 80℃, respectively, comparing to those of 0.14 mS·cm-1 and 1.20 mS·cm-1 for the po-FPA membrane. Furthermore, the mechanical strength of 9.9 MPa was obtained for the Li-SGO-po-FPAS, which is acceptable for the application in Li-ion batteries. The electrochemical characterizations indicate the better compatibility between the single ion conducting polymer electrolyte and the electrode interface after doping with the Li-SGO. The Li-SGO-po-FPAS showed the lithium ion transference number of 0.91 and electrochemical window of 4.6 V vs. Li+/Li. The Li|LiFePO4 Li-ion battery assembled from the Li-SGO-po-FPAS exhibited good cyclability and higher C-rate capacity. The results suggest that the treatment of GO by lithiation and sulfonation processes is useful for application in single ion conducting polymer electrolyte, and it is also favorable for improving the comprehensive performance of single ion conducting polymer electrolyte, subsequently superior battery performance.

/
| 〈 |
|
〉 |