

石榴石固体电解质Li3BO3界面改性研究
收稿日期: 2020-05-15
修回日期: 2020-06-22
网络出版日期: 2020-06-28
基金资助
国家重点研发计划项目(2018YFB0905400);国家自然科学基金项目(21875196);国家自然科学基金项目(21935009);国家自然科学基金项目(U1732121);福建省引导性计划项目(2019H0003);厦门大学大学生创新创业训练计划项目(S201910384404)
Study on Li3BO3 Interface Modification of Garnet Solid Electrolyte
Received date: 2020-05-15
Revised date: 2020-06-22
Online published: 2020-06-28
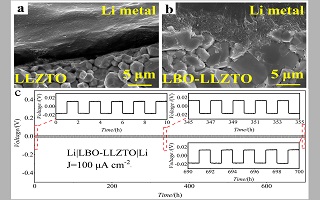
石榴石固体电解质由于其高的离子电导率,对锂金属稳定等优点成为了下一代高性能锂电池的重要研究方向之一。但锂金属负极界面浸润性与锂枝晶问题限制了其应用。本文通过简单的液相沉积结合高温烧结的方法,在石榴石固体电解质片表面构建了一层稳定的硼酸三锂(Li3BO3)修饰层。研究表明,Li3BO3修饰层可以有效改善石榴石固体电解质与锂金属负极界面接触,促进锂的均匀沉积/溶出,从而抑制锂枝晶生长,提高界面稳定性。Li3BO3修饰后石榴石电解质片与锂金属之间紧密结合,Li/石榴石界面阻抗由修饰前的1780 Ω·cm2降低至58 Ω·cm2。得益于界面接触的改善,Li3BO3修饰后的LLZTO电解质组装的对称电池可以在0.1 m·cm-2的电流密度下稳定工作超过700 h。而未修饰的对称电池在0.05 mA·cm-2的电流密度下短时间工作即出现微短路现象。
陈规伟 , 龚正良 . 石榴石固体电解质Li3BO3界面改性研究[J]. 电化学, 2021 , 27(1) : 76 -82 . DOI: 10.13208/j.electrochem.200516
Garnet solid-state electrolytes have become the research hotspot due to their high ionic conductivity, wide electrochemical stability window and good air stability. However, there are still a series of problems to be solved. The poor contact between the lithium (Li) metal and garnet pellet make it difficult to build stable ion diffusion channels, resulting in large interfacial resistance. The continuous growth of lithium dendrites can penetrate the electrolyte pellet and cause a short circuit in the solid-state battery. Herein, a novel strategy is proposed to improve the wettability of LLZTO electrolyte with Li metal, via interfacial modification of LLZTO electrolyte with tri-lithium borate (Li3BO3). Li3BO3 is chemically stable with Li metal and effective to improve the wettability between Li and LLZTO pellet. A stable and even Li3BO3 interfacial layer was constructed on the LLZTO electrolyte surface by liquid-phase deposition combing with high temperature sintering. The low melting point (700℃) of Li3BO3 facilitated the formation of a dense and uniform coating layer. SEM images show that the Li3BO3 layer was about 2.5 μm thick and completely covered the pellet surface. Intimate contact between Li metal and LLZTO electrolyte could be realized after the Li3BO3 interfacial modification, which was confirmed by SEM analysis and wettability experiment. Benefiting from the significantly improved interfacial contact, the interfacial impedance was dramatically reduced from 1780 Ω·cm2 of Li/LLZTO interface to 58 Ω·cm2 of Li/LBO-LLZTO interface. The Li|LBO-LLZTO|Li symmetric cell could produce a low overpotential and work stably at the current density of 0.1 mA·cm-2 for more than 700 h. By contrast, the Li|LLZTO|Li symmetric cell displayed high overpotential and was short circuited after 20 min of lithium plating/stripping at the current density of 0.05 mA·cm-2. Our results show that Li3BO3 interfacial modification is an effective approach to improve the wettability and interfacial stability between Li metal and garnet electrolyte, which is a key to the successful use of solid-state battery.

/
| 〈 |
|
〉 |