

还原氧化石墨烯@泡沫镍载CoO纳米花电极制备及催化CO2性能
收稿日期: 2020-05-12
修回日期: 2020-06-03
网络出版日期: 2020-06-28
基金资助
江苏省大学生创新训练项目;江苏省科技厅面上项目(BK20181485);连云港市“521”项目(LYG52105-2018038);“江苏省高校一流本科专业建设项目”;“江苏省重点学科建设项目”;江苏省高校优势学科建设工程资助项目
Preparation of CoO/RGO@Ni Foam Electrode and Its Electrocatalytic Reduction of CO2
Received date: 2020-05-12
Revised date: 2020-06-03
Online published: 2020-06-28
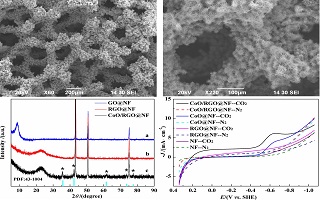
本文以氧化石墨烯包覆泡沫镍电极(GO@NF)作为基底,采用水热法在GO@NF基底上原位生长CoO纳米花,同时GO在水热过程中被同步热还原为还原氧化石墨烯(RGO),从而一步制得还原氧化石墨烯包覆泡沫镍负载CoO纳米花电极(CoO/RGO@NF)。使用XRD和SEM对CoO/RGO@NF电极进行表征,发现CoO纳米花均匀生长在泡沫镍三维网络结构上,CoO纳米花为大量针状纳米棒围绕一个中心而成的花状结构,纳米棒的长度约为10 ~ 15 μm,直径约为100 ~ 200 nm。使用循环伏安和线性扫描法测试了CoO/RGO@NF电极电催化CO2的还原性能,在-0.76 V(vs. SHE)电位下,CoO/RGO@NF电极电催化CO2还原的电流效率达到70.9%,产甲酸法拉第效率达到65.2%,甲酸产率为59.8 μmol·h-1·cm-2,且电极可持续稳定电催化还原CO2 4 h,表明CoO/RGO@NF电极对CO2电还原有着优良的催化活性、选择性和稳定性。
郭茜 , 富佳龙 , 张成燕 , 蔡超越 , 王城 , 周丽华 , 许瑞波 , 王明艳 . 还原氧化石墨烯@泡沫镍载CoO纳米花电极制备及催化CO2性能[J]. 电化学, 2021 , 27(4) : 449 -455 . DOI: 10.13208/j.electrochem.200513
The worldwide extensive release of carbon dioxide (CO2) has caused serious environmental pollution and unprecedented climate change problems. Thus, for the sustainable development of human society, it is very necessary to convert CO2 to renewable fuels through clean and economical processes. The electrochemical CO2 reduction reaction (CO2RR) is regarded as a promising approach for the recycling of carbon resource and the generation of sustainable fuels. However, the slow kinetics and formation of multiple products in CO2RR hinder its large-scale application. Hence, great research efforts are made to develop electrocatalysts with high product selectivity at low overpotential. Recently, nanostructured transition metal oxide based electrocatalysts have displayed quite exciting performances for the CO2RR, in terms of fast kinetics, selectivity and durability. Among the various metal oxides, cobalt oxides show high CO2RR activity, and selective for the formation of formic acid. In this paper, a hybrid of CoO nanoflowers grown onto three-dimensional (3D) reduced graphene oxide (RGO)@Ni foam (CoO/RGO@NF) was synthesized by a facile hydrothermal method. The composite electrode of CoO/RGO@NF was characterized by XRD and SEM. It is found that the CoO nanoflowers grew uniformly on the 3D network of RGO@NF electrode. The CoO nanoflowers were formed by a large number of nanorods around a center. The length of the nanorods was about 10 ~ 15 μm, and the diameter was about 100 ~ 200 nm. The electrocatalytic performance of CoO/RGO@NF composite electrode for CO2 reduction was studied by cyclic voltammetry and linear scanning voltammetry. The results showed that the current efficiency of CoO/RGO@NF electrode for electrocatalytic reduction of CO2 was 70.9% and the Faraday efficiency for formic acid production was 65.2%. In addition, the yield of formic acid on the electrode was 59.8 μmol·h-1·cm-2 at -0.76 V(vs. SHE) after 4 h of electrolysis. Furthermore, the current density was stable at about 90%. These data indicated that the as-prepared CoO/RGO@NF composite electrode had achieved excellent catalytic activity, selectivity and stability for CO2 electroreduction.

Key words: CO2 electrocatalysis; hydrothermal method; nickel foam; CoO nanoflowers
/
| 〈 |
|
〉 |