

Co@NC纳米复合材料的制备及其电化学性能研究
收稿日期: 2019-05-06
修回日期: 2019-08-10
网络出版日期: 2019-10-28
基金资助
浙江省自然科学基金杰出青年科学基金项目(No. LR17B060003)资助
Preparations and Electrochemical Properties of Co@NC Nanocomposites Based on ZIF-67
Received date: 2019-05-06
Revised date: 2019-08-10
Online published: 2019-10-28
谭小莉 , 何灯红 , 张兴旺 . Co@NC纳米复合材料的制备及其电化学性能研究[J]. 电化学, 2019 , 25(5) : 601 -607 . DOI: 10.13208/j.electrochem.181146
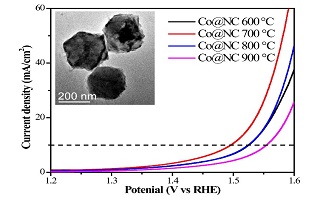
Key words: ZIF-67; Co@NC; nanocomposites; electrocatalytic properties
/
| 〈 |
|
〉 |