

一种简单灵敏的基于适配体的黄曲霉毒素B1电化学传感器
收稿日期: 2019-02-19
修回日期: 2019-04-11
网络出版日期: 2019-06-07
基金资助
国家重点研发计划项目(No. 2018YFC1604000)资助
A Simple and Sensitive Aptamer-Based Electrochmical Sensor for Determination of Aflatoxin B1
Received date: 2019-02-19
Revised date: 2019-04-11
Online published: 2019-06-07
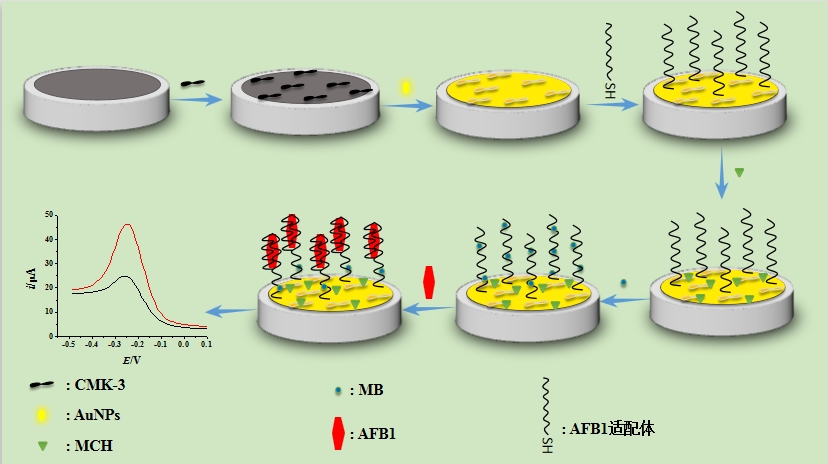
黄曲霉毒素B1(AFB1)以其高毒性和致癌性成为食品安全隐患而备受关注. 本文拟构建一种新颖、简单、快速、灵敏的传感器用于谷物食品中AFB1的痕量检测. 将介孔碳(CMK)修饰在工作电极表面来增大电极的表面积,再将工作电极恒电位沉积金纳米粒子(AuNPs),提高电信号的同时,为下一步巯基化适配体的连接提供位点. 检测过程中,AFB1可以竞争性地去除吸附在适配体链上的亚甲基蓝(MB)引起电信号的变化,对AFB1进行定量检测. 修饰的工作电极导电性能得到改善,灵敏度大大提高,对AFB1的线性响应范围为0.1 ~ 75 μg·L-1,检出限低至36 ng·L-1. 在对不同谷物食品(大米、玉米、糯米)进行加标回收实验中,回收率在92.3% ~ 103.6%范围之间,实现对目标物的定量检测. 本文为食品中AFB1快速检测方法提供了一种新思路和新方法.
刘冰 , 赵耀帅 , 秦月 , 王怡 , 朱艳杰 , 王硕 . 一种简单灵敏的基于适配体的黄曲霉毒素B1电化学传感器[J]. 电化学, 2020 , 26(3) : 422 -430 . DOI: 10.13208/j.electrochem.190219
Aflatoxin B1 has attracted much attention because of its high toxicity and carcinogenicity, which has become a great concern in food safety. Based on the principle of specific binding between Aflatoxin B1 and its aptamer, an aptamer-based electrochemical sensor had been designed and developed for the determination of minor Aflatoxin B1 contained in grain. The mesoporous carbons were first modified on the surface of the working electrode, and then the gold nanoparticles were on-site electrodeposited at a constant potential. Each modified electrode was characteritised by scanning electron microscopy (SEM) and electrochemical impedance spectroscopy (EIS). As a result, the surface area and the electrochemical signal of the modified electrode were all greatly increased, providing more attachment sites for the following conjugation of the aptamer. During the detecting process, Aflatoxin B1 could compete with methylene blue on the aptamer chain to cause methylene blue shedding and the electrochemical signals were changed which could be used to quantify the concentration of Aflatoxin B1. The surface modifications could evidently improve the conductivity and sensitivity of the sensor. A linear response in current to Aflatoxin B1 was found ranging from 0.1 to 75 μg·L-1 with the detection limit as low as 36 ng·L-1 (S/N = 3). The spiked recovery tests of different grains (rice, corn, glutinous rice) revealed that the recovery rates were between 92.3 and 103.6%, showing excellent accuracy, sensitive quantitative detection of the target substance and good reproducibility. This work has demonstrated a new method to develop a novel, simple, fast and sensitive sensor for the detection of trace amount of Aflatoxin B1 in grains.

Key words: Aflatoxin B1; aptamer; sensor; detection method
/
| 〈 |
|
〉 |