

间歇电沉积法制备纳米MnOx/Ti膜电极及其催化氧化环己烷性能
收稿日期: 2019-03-12
修回日期: 2019-04-18
网络出版日期: 2019-04-25
基金资助
国家自然科学基金(No. 21676200,No. 21576208)、教育部创新团队发展计划(No. IRT-17R80)和天津科学支撑计划(No. 17JCYBJC19800)
Preparations of Nano-MnOx/Ti Electrocatalytic Membrane Electrode for Catalytic Oxidation of Cyclohexane Using Intermittent Electrodeposition
Received date: 2019-03-12
Revised date: 2019-04-18
Online published: 2019-04-25
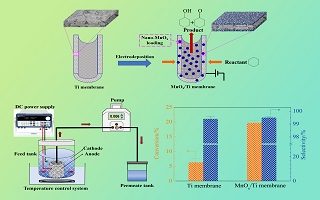
高选择性氧化环己烷(CHA)制备环己酮和环己醇(KA油)具有重要的工业价值和应用前景. 本文提出采用间歇电沉积法制备纳米MnOx催化剂负载多孔管式钛膜,构建电催化膜反应器(ECMR)催化氧化环己烷制备环己醇和环己酮. 利用场发射扫描电子显微镜(FESEM)、X射线衍射仪(XRD)和电化学工作站等表征手段对催化剂的结构与性能进行表征. 结果表明,间歇电沉积法制备的催化剂为纳米花球状γ-MnO2. 与基体钛膜相比,MnOx/Ti膜电极具有更优的电化学性能和传质性能. 此外,以MnOx/Ti电催化膜为阳极,不锈钢网为阴极构建ECMR. 当环己烷初始浓度30 mmol·L-1、反应温度30oC、停留时间34.3 min、电流密度2.3 mA·cm-2等条件下,ECMR环己烷转化率达25.6%,KA油总选择性高于99%. 同时,ECMR重复使用8次后表现较高催化稳定性.
关键词: 间歇电沉积; MnOx/Ti电催化膜; 电催化膜反应器; 环己烷氧化; 环己醇和环己酮
周雪 , 王虹 , 尹振 , 张玉军 , 李建新 . 间歇电沉积法制备纳米MnOx/Ti膜电极及其催化氧化环己烷性能[J]. 电化学, 2020 , 26(3) : 397 -405 . DOI: 10.13208/j.electrochem.190312
Cyclohexanone and cyclohexanol (KA oil) obtained from highly selective oxidation of cyclohexane (CHA) show important industrial value and application prospects. In this work, the intermittent electrodeposition was developed to prepare nano-MnOx catalyst loading porous tubular titanium membrane electrode (MnOx/Ti), which was employed to constitute an electro-catalytic membrane reactor (ECMR) for the oxidation of cyclohexane to produce cyclohexanol and cyclohexanone. The surface morphology, crystal structure and electrochemical property of the catalysts were characterized by FESEM, XRD and electrochemical workstation, respectively. The results show that the catalyst prepared by the intermittent electrodeposition displayed nano-flower-like γ-MnO2. Compared with titanium membrane, the MnOx/Ti electrocatalytic membrane exhibited better electrochemical performance and mass transfer performance. Furthermore, the ECMR was constructed by using the MnOx/Ti electrocatalytic membrane as the anode and the stainless steel mesh as the cathode. When the initial concentration of cyclohexane was 30 mmol·L-1, the reaction temperature was 30oC, the residence time was 34.3 min and the current density was 2.3 mA·cm-2. The cyclohexane conversion rate reached 25.6% and the total selectivity of KA oil exceeded 99%. Simultaneously, the ECMR with MnOx/Ti electrode showed a good stability during the oxidation of cyclohexane.

/
| 〈 |
|
〉 |