

间接电氧化2-丁酮制备α-羟基缩酮的研究
收稿日期: 2019-02-25
修回日期: 2019-04-01
网络出版日期: 2019-04-23
基金资助
国家重点研发计划项目(No. 2017YFB0307502)资助
Preparation of α-Hydroxylated Acetal from 2-Butanone by Indirect Electrooxidation
Received date: 2019-02-25
Revised date: 2019-04-01
Online published: 2019-04-23
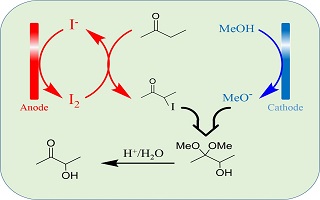
在板框式循环电解槽中,以KOH为电解质,KI为催化剂,石墨电极分别为阳极和阴极,研究电化学间接氧化2-丁酮合成乙偶姻中间体α-羟基缩酮,讨论电流密度、极板间电解液流速、电解液中2-丁酮浓度、电解温度以及通电量等电解条件对中间体收率和电流效率的影响,经优选工艺条件为:电流密度40 mA·cm-2,流速6.4 cm·s-1,2-丁酮浓度1.75 mol·L-1,电解温度30℃,通电量为1.5 F·moL-1时,中间体收率可达78.9%,电流效率40.1%. 循环伏安测试结果表明,电解时碘离子在阳极氧化生成碘单质,甲醇在阴极还原生成甲氧基负离子,原料2-丁酮与电解产物反应,并最终生成乙偶姻中间体.
黄海清 , 缪烨 , 张新胜 . 间接电氧化2-丁酮制备α-羟基缩酮的研究[J]. 电化学, 2020 , 26(3) : 389 -396 . DOI: 10.13208/j.electrochem.190222
Acetoin (3-hydroxy-2-butanone) is an important food spice. As a platform compound, it is widely used in medicine, tobacco, cosmetics, chemical material and other industries. In this paper, α-hydroxylated acetal, an intermediate of acetoin, was prepared from 2-butanone by indirect electrooxidation in the plate and frame electrolytic cell, in which graphite plates were used as both an anode and a cathode, while KOH as an electrolyte and KI as a catalyst. Acetoin could be prepared by hydrolysis in acidic aqueous solution from acetoin intermediate. The effects of current density, electrolyte flow rate between the plates, 2-butanone concentration and electrolysis temperature on the yield and efficiency of acetoin intermediate were investigated. Under the optimized conditions, namely, the current density of 40 mA·cm-2, the flow rate of 6.4 cm·s-1, the 2-butanone concentration of 1.75 mol·L-1, the electrolysis temperature of 30 °C, and the passed charge of 1.5 F·mol-1, the yield and current efficiency of the acetoin intermediate could reach 78.9% and 40.1%, respectively. The cyclic voltammetric tests showed that during electrolysis, iodine ions were oxidized to iodine on the anode, while methanol was reduced to methoxy anion on the cathode. 2-butanone reacted with the electrolytic products, and eventually, the acetoin intermediate was formed.

/
| 〈 |
|
〉 |