

膜电极构型CO2还原电解单池的稳定性研究
收稿日期: 2019-03-05
修回日期: 2019-04-08
网络出版日期: 2019-04-12
基金资助
辽宁省自然科学基金项目(No. 201602162)、大连理工大学GF创新基金项目(No. DUT18GF308)和国家电网公司科技项目(No. SGRI-DL-71-16-015)资助
Stability Studies for a Membrane Electrode Assembly Type CO2 Electro-Reduction Electrolytic Cell
Received date: 2019-03-05
Revised date: 2019-04-08
Online published: 2019-04-12
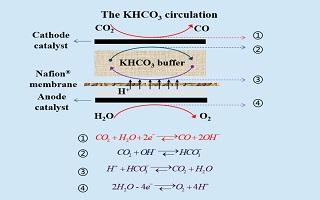
电化学还原CO2可实现CO2的资源化转化,是缓解因其过度排放所导致诸多环境问题的关键技术. 本文提出了一种膜电极(membrane electrode assembly,MEA)构型CO2还原电解单池的结构设计,可同步实现气体扩散阴极两侧CO2的供给与电解质液层的更新. 基于该MEA构型电解池,实验考察了电解质液层中KHCO3浓度和更新与否对氮掺杂石墨烯锚定的Ni电极表面CO2电还原制备CO的反应活性、产物分布与稳定性的影响. 结果表明,若电流密度低于5 mA·cm-2,KHCO3浓度显著影响电解电势而非产物分布. CO2还原电解单池在稳定运行中存在着“可逆”与“不可逆”两种衰减模式. 其中,阴极/电解质界面处催化剂的流失是 “不可逆”衰减形成的原因;而电解质液层中KHCO3溶液的流失导致了MEA构型CO2还原单池的“可逆”衰减,周期性更新KHCO3电解质是降低其“可逆”衰减的有效方法.
毛庆 , 李冰玉 , 景维云 , 赵健 , 刘松 , 黄延强 , 杜兆龙 . 膜电极构型CO2还原电解单池的稳定性研究[J]. 电化学, 2020 , 26(3) : 359 -369 . DOI: 10.13208/j.electrochem.190305
Electro-catalytic reduction is an efficient way to achieve resourcable transformation of CO2, which is one of the important techniques to solve the global environmental problems originated from excessive CO2 emission. In this study, a membrane electrode assembly(MEA) type CO2 electro-reduction electrolytic cell was constucted, which enables CO2 feeding and real-time KHCO3 aqueous updating on both sides of the cathode gas diffusion electrode (GDE). By means of the electrolytic cell, effects of KHCO3 concentration and updating inside the liquid electrolytic chamber on CO2 electro-reduction activity, production distribution and stability were investigated. The experimental results suggested that the KHCO3 concentration exerted strong influence on the cell voltage rather than the production distribution for the current densities lower than 5 mA·cm-2. The performance of MEA type CO2 electro-reduction cell decayed in both “reversible” and “irreversible” ways. Catalysts leaking at the GDE/liquid electrolyte interface might be respossible for the cell “irreversible” decay. Meanwhile, th leakage of KHCO3 aqueous electrolyte arose from gas accumulation in the liquid electrolytic chamber contributed to the “reversible” degradation, which could be recovered effectively by updating the KHCO3 aqueous electrolyte.

/
| 〈 |
|
〉 |