

中空核壳结构Ni1.2Co0.8P@N-C钠离子电池负极材料的制备及拉曼研究
收稿日期: 2019-03-18
修回日期: 2019-04-05
网络出版日期: 2019-04-08
基金资助
国家自然科学基金项目(No. 21775127)、福建省自然科学基金项目(No. 2018J05025)、厦门市科技局产学研协同创新及合作项目(No. 3502Z20173017)资助
Synthesis and Raman Study of Hollow Core-Shell Ni1.2Co0.8P@N-C as an Anode Material for Sodium-Ion Batteries
Received date: 2019-03-18
Revised date: 2019-04-05
Online published: 2019-04-08
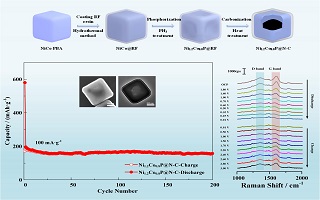
本文设计制备了一种新型的氮掺杂碳包覆镍钴双金属磷化物中空核壳结构纳米立方体(Ni1.2Co0.8P@N-C)作为钠离子电池负极材料. 该材料以镍钴类普鲁士蓝(PBA)纳米粒子为模板,先后经水热法、磷化法和高温碳化处理后合成. 将其作为活性材料应用在钠离子电池中,该材料展现出优异的循环稳定性,当以100 mA·g-1的电流密度循环至200圈时,该材料的库仑效率保持在99.3%. 进一步通过对不同电位下Ni1.2Co0.8P@N-C材料中的氮掺杂碳进行原位拉曼光谱测试,结果显示钠离子在氮掺杂的碳壳中的脱嵌行为具有较大程度的可逆性,研究结果对钠离子电池充放电过程的后续电化学研究提供了有价值的信息.
陈嘉卉 , 钟晓斌 , 何超 , 王晓晓 , 许清池 , 李剑锋 . 中空核壳结构Ni1.2Co0.8P@N-C钠离子电池负极材料的制备及拉曼研究[J]. 电化学, 2020 , 26(3) : 328 -337 . DOI: 10.13208/j.electrochem.190318
With the increasing demand for large-scale energy storage, great progress has been made in discovering new advanced energy storage materials. Sodium-ion batteries (SIBs) have attracted much attention in recent years due to their use of abundant sodium resources and their comparable electrochemical capacity to lithium-ion batteries (LIBs). In this paper, we developed novel hollow core-shell Ni-Co bimetallic phosphide nanocubes with N-doped carbon coatings (Ni1.2Co0.8P@N-C) as the anode material for SIBs. The material was synthesized through a low-temperature phosphorization method using resorcinol formaldehyde (RF) resin coating with a Ni-Co Prussian blue analogue (PBA) as a template and a subsequent thermal annealing process. The size of the as-obtained nanocubes was about 310 nm with a 19 nm N-doped carbon shell. When used as the anode material of SIBs, Ni1.2Co0.8P@N-C exhibited the excellent electrochemical cycling stability and demonstrated an especially high coulombic efficiency of 99.3%, even after 200 cycles with current density of 100 mA·g-1. Furthermore, in-situ Raman spectroscopy was used to investigate the electrode material in order to understand the electrochemical processes in the N-doped carbon shell of Ni1.2Co0.8P@N-C. The results showed that the intercalation and de-intercalation behavior of sodium ions in the N-doped carbon shell was almost reversible, providing valuable information about the charge and discharge processes in SIBs for the follow-up electrochemical studies.

/
| 〈 |
|
〉 |