

Ni-Fe/Ti和Ni-Fe-S/Ti的制备及其电催化水分解性能
收稿日期: 2019-01-14
修回日期: 2019-04-01
网络出版日期: 2019-03-29
基金资助
国家自然科学基金项目(21706010);江苏省自然科学基金面上项目(BK20161200)
版权
Preparations of Nickel-Iron Hydroxide/Sulfide and Their Electrocatalytic Performances for Overall Water Splitting
Received date: 2019-01-14
Revised date: 2019-04-01
Online published: 2019-03-29
Copyright
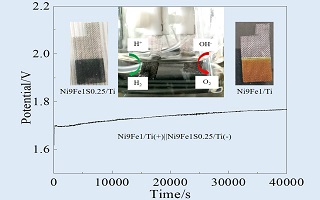
以钛网为基底,采用电沉积法制备了Ni-Fe/Ti析氧电极,然后将得到的Ni-Fe/Ti电极通过固相硫化制备了Ni-Fe-S/Ti析氢电极. 分别考察了电沉积液中Ni2+/Fe3+离子摩尔浓度比和硫脲加入量对Ni-Fe/Ti和Ni-Fe-S/Ti结构和电化学性能的影响. 结果表明,随着电沉积液中Ni2+含量的增加,Ni-Fe/Ti电极析氧性能先增强后减弱,Ni9Fe1/Ti电极具有最好的析氧性能;随着硫脲加入量的增加,Ni-Fe-S/Ti电极析氢性能呈现先增强后减弱的趋势,Ni9Fe1S0.25/Ti电极具有最好的析氢性能. 在50 mA·cm-2下,Ni9Fe1/Ti电极的析氧过电位为280 mV,Ni9Fe1S0.25/Ti电极的析氢过电位为269 mV,且均具有很好的稳定性. 将Ni9Fe1/Ti与Ni9Fe1S0.25/Ti分别作为阳极和阴极进行电催化全水分解,电流密度达到50 mA·cm-2所需电势仅1.69 V,表现出很好的全水解催化性能.
陆杭烁 , 何小波 , 银凤翔 , 李国儒 . Ni-Fe/Ti和Ni-Fe-S/Ti的制备及其电催化水分解性能[J]. 电化学, 2020 , 26(1) : 136 -147 . DOI: 10.13208/j.electrochem.190114
The Ni-Fe/Ti oxygen evolution electrode was prepared by electrodeposition on a titanium mesh substrate. Then, the as prepared Ni-Fe/Ti electrode was used to derive the Ni-Fe-S/Ti hydrogen evolution electrode through solid phase sulfuration. The effects of the molar ratio of Ni2+ to Fe3+ in the electrolyte and the amount of thiourea on the structures and electrochemical performances of Ni-Fe/Ti and Ni-Fe-S/Ti electrodes were investigated. The results show that the oxygen evolution performance of Ni-Fe/Ti electrode was first increased and then decreased with the increase of nickel ion content in the electrolyte. The Ni9Fe1/Ti electrode exhibited the best oxygen evolution performance. With the increase of thiourea addition, the hydrogen evolution performance of Ni-Fe-S/Ti electrode was increased firstly and then decreased. The Ni9Fe1S0.25/Ti electrode showed the best hydrogen evolution performance. To achieve a current density of 50 mA·cm-2, an overpotential of 280 mV was required for oxygen evolution reaction (OER) with the Ni9Fe1/Ti electrode, while 269 mV for hydrogen evolution reaction (HER) with the Ni9Fe1S0.25/Ti electrode, both with good stabilities. Accordingly, the Ni9Fe1/Ti and Ni9Fe1S0.25/Ti electrode were used as anodes and cathodes, respectively, for overall water splitting tests. The current density of 50 mA·cm-2 was achieved at a voltage of 1.69 V, showing the good catalytic performance of overall water splitting.

/
| 〈 |
|
〉 |