

基于CoP纳米阵列的亚硝酸根传感器的研究
收稿日期: 2019-01-02
修回日期: 2019-01-26
网络出版日期: 2019-01-29
基金资助
国家自然科学基金项目(No. 21575137)资助
High-Efficiency Nitrite Sensor Based on CoP Nanowire Array
Received date: 2019-01-02
Revised date: 2019-01-26
Online published: 2019-01-29
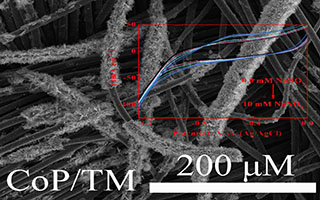
周福玲 , 熊小莉 , 孙旭平 . 基于CoP纳米阵列的亚硝酸根传感器的研究[J]. 电化学, 2019 , 25(2) : 252 -259 . DOI: 10.13208/j.electrochem.181055

Key words: CoP; nanoarray; nitrite; electrochemical; sensor
/
| 〈 |
|
〉 |