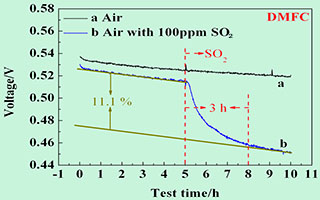
直接甲醇燃料电池(DMFC)通常采用空气中氧气作为氧化剂,但空气中硫化物、氮化物等污染物会对电池性能造成影响. 本文采用恒流放电曲线、极化曲线、循环伏安扫描(CV)和电化学阻抗谱(EIS)等方法,研究SO2对DMFC电池性能影响,分析其毒化作用机制. 研究表明,SO2毒化导致催化剂电化学活性面积(ECSA)减小,氧还原反应(ORR)电荷转移电阻增大,从而造成DMFC电池开路电压和工作电压加速衰减,峰值功率密度减小. 进一步探究了三种恢复策略,空气吹扫与I-V变载操作都只能实现电池性能的部分恢复,CV扫描可完全恢复电池性能.
Direct methanol fuel cells (DMFC) generally use oxygen as an oxidant. Contaminants such as sulfides and nitrides in the air can affect the performance of the DMFC. In this work, the effects of SO2 on the performance of DMFC were investigated and the mechanism of poisoning was analyzed, by means of constant current discharge curve, polarization performance curve, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). In the CV scan, the permeated methanol was oxidized at a low potential to eliminate its effect on the SO2 poisoning behavior test. The results showed that the SO2 poisoning resulted in a decrease in the electrochemical activity surface area (ECSA) of the catalyst. Meanwhile, the EIS data indicated that the poisoning led to an increase in the charge transfer resistance of the oxygen reduction reaction (ORR). Therefore, the poison accelerated decay of the open circuit voltage and operating voltage of the DMFC, and decreased the peak power density. Further investigations of three recovery strategies, dry air purging and load-shifting I-V operations could only partially restore the performance of DMFC. However, CV scanning could accomplish the recovery more completely.