

负载于刻蚀镍泡沫上钯纳米粒子作为乙醇氧化高性能电催化剂
收稿日期: 2018-11-12
修回日期: 2018-12-20
网络出版日期: 2019-01-10
Pd Nanoparticles Supported on the Etched Ni Foams as High-Performance Electrocatalysts for Direct Ethanol Fuel Cells
Received date: 2018-11-12
Revised date: 2018-12-20
Online published: 2019-01-10
Supported by
This work was supported by the National Basic Research Program of China (2015CB932304 and 2016YFA0202603), NSFC (91645104), Science and Technology Program of Guangzhou (201704030019), Natural Science Foundation of Guangdong Province (2016A010104004 and 2017A010103007), and Guangdong Science and Technology Innovation Leading Talent Fund (2016TX03N187).
张 翅 , 李成飞 , 李高仁 . 负载于刻蚀镍泡沫上钯纳米粒子作为乙醇氧化高性能电催化剂[J]. 电化学, 2019 , 25(5) : 571 -578 . DOI: 10.13208/j.electrochem.181144
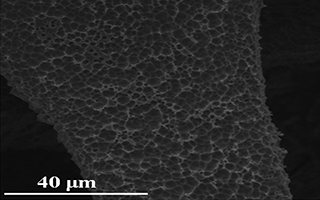
Key words: Pd nanoparticle; Ni foam; mixed acids etching; electrocatalyst; ethanol oxidation
/
| 〈 |
|
〉 |