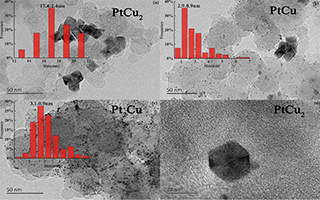
利用溶剂热法,在N,N-二甲基甲酰胺(DMF)溶剂中共同还原乙酰丙酮铂(Pt(acac)2)和乙酰丙酮铜(Cu(acac)2)制备PtCu八面体合金催化剂. PtCu2八面体表现出明显的晶格收缩、较高比例的非氧化态Pt单质和较高的电子结合能,进而表现出较弱的含氧物种吸附强度和较低的d 带中心位置. 系统研究结构导向剂对PtCu合金形貌影响. 在半电池测试中,由于PtCu2具有均匀分散的规则八面体形貌结构,导致在0.9 V vs. RHE处氧还原(ORR)的质量比活性和面积比活性分别是Pt/C(JM)的6.3和27.2倍,并在加速衰减测试后其ORR的质量比活性仍达到Pt/C(JM)的4.5倍.
Platinum acetylacetonate (Pt(acac)2) and copper acetylacetonate (Cu(acac)2) were co-reduced to prepare PtCu2 octahedron alloy catalyst in N,N-dimethylformamiade by solvothermal method. The PtCu2 showed lattice compression, and high ratio of non-oxidized Pt with high electronic binding energy. All those structural features contributed to weak adsorption strength of oxygen species on Pt and lower d-band centre position. The influence of structure-directing agent on morphology of PtCu alloy was systematically studied. In the half cell test, as a result of the uniform morphology and regular octahedron of PtCu2 formed, the mass activity and area specific activity of PtCu2/C reached 6.2 and 27.2 times, respectively, relative to those of Pt/C at 0.9 V vs. RHE. Furthermore, after the accelarated degradation test, the mass activity of PtCu2/C still reached 4.5 times compared to that of Pt/C.