

前驱体中N含量对FeN/C催化剂氧还原活性的影响研究
收稿日期: 2018-09-23
修回日期: 2019-02-16
网络出版日期: 2020-02-28
基金资助
国家自然科学基金(21506041);贵州省科学技术基金(黔科合JZ字[2015]2007号);贵州省留学人员科技活动择优资助项目(黔人项目资助合同(2014)12号);贵州师范大学资助博士科研项目资助
版权
Effect of Nitrogen Content in Catalyst Precursor on Activity of FeN/C Catalyst for Oxygen Reduction Reaction
Received date: 2018-09-23
Revised date: 2019-02-16
Online published: 2020-02-28
Copyright
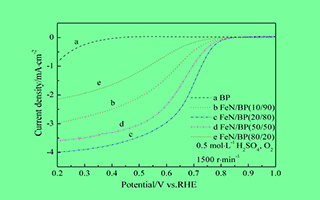
采用热解法制备FeN/C催化剂,考察催化剂前驱体中氮含量对其氧还原活性的影响. 使用X射线衍射、比表面积和孔径分布测试、透射电子显微镜以及热重分析等方法对催化剂的结构、形貌及催化剂前驱体的热性质等进行表征,使用线性扫描伏安法对催化剂的氧还原活性进行测试. 结果表明,以1,10-菲啰啉为氮源,FeCl3为铁源,Black Pearl 2000为载体,催化剂前驱体中1,10-菲啰啉含量为20wt%,Fe含量为1wt %时,热处理制备所得催化剂粒子分布均匀,比表面积为824.48 m2·g-1,平均孔隙为10.58 nm,表面的氮元素含量为0.31wt%;并具有最好的氧还原催化活性.催化剂前驱体中氮源含量在热解过程中导致催化剂的比表面积、孔径结构及表面氮元素含量的变化是影响催化剂活性的关键因素.
杨智 , 沈亚云 , 周娥 , 魏成玲 , 秦好丽 , 田娟 . 前驱体中N含量对FeN/C催化剂氧还原活性的影响研究[J]. 电化学, 2020 , 26(1) : 130 -135 . DOI: 10.13208/j.electrochem.180923
Non-noble metal catalysts with high activity and low cost have attracted increasing interest as potential catalysts for oxygen reduction reaction (ORR) to replace Pt-based catalysts. In this paper, the effect of nitrogen content in catalyst precursor on ORR activity of FeN/C catalyst was investigated by X-ray diffraction (XRD), Brunauer-Emmet-Teller (BET) surface area and pore size distribution measurements, transmission electron microscope (TEM), thermogravimetric analysis (TGA), and rotating disk electrode (RDE) techniques. The results show that the most active catalyst was obtained by pyrolysis in argon at 1050 °C with a catalyst precursor containing 20wt% 1,10-phenanthroline, 1wt% Fe and Black Pearl 2000. The particle size and distribution, BET surface area and pore size distribution, surface nitrogen content are key factors affecting the catalytic activity of catalyst. The difference in ORR activities may be explained by TGA data of catalyst precursors with different nitrogen contents, where the pyrolysis of catalyst precursor with phen/BP ratio of 20/80 resulted in weight loss of 28.1% at the temperature above 420 °C, which may generate most of the catalytic sites for ORR.

/
| 〈 |
|
〉 |