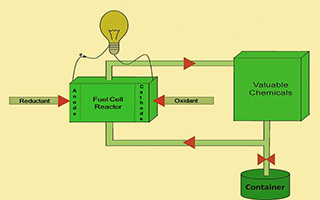
燃料电池作为能源转换装置能够高效地将化学能转化为电能,随着技术的发展人们将其作为反应器来完成高附加值的化学品的合成,同时产生一定的电能. 燃料电池反应器因具有反应条件温和、反应过程可控、产物选择性高、能源利用效率高等特点,而被广泛地应用于医药中间体的制备、气体分离、水处理等多个领域. 本文首先按照反应器中阴阳极区域发生反应的类型进行分类,介绍燃料电池反应器在化学品与电能联产中的研究现状和研究进展. 随后描述了燃料电池反应器中存在的问题,并依照催化剂、反应过程等方向对解决方案进行探讨. 最后,对几种新型燃料电池反应器的研究进行了简要的介绍并对其发展做出了展望.
As an energy conversion device, fuel cells can efficiently convert chemical energy into electrical energy. With the developing of technology, it is used as a reactor to conduct the synthesis of high value-added chemicals while generating electrical energy. Having benefits such as mild reaction conditions, controllability of the reaction process, high selectivity of the product, as well as high efficiency of energy utilization, it is widely used in many fields such as preparation of high value-added industrial products, gas separation, water treatment, etc. This paper introduces the current trends and statuses of fuel cell reactors in the cogeneration of chemicals and electric energy according to the reduction reaction at the cathode and the oxidation reaction of the anode. The problems related to the fuel cell reactor are described, and possible solutions are discussed in terms of the catalyst research, process research and others. Finally, the research in several new fuel cell reactors is briefly introduced and its development is prospected.