

石榴石型固态电解质/铝锂合金界面构筑及电化学性能
收稿日期: 2018-10-01
修回日期: 2018-11-29
网络出版日期: 2018-11-29
基金资助
国家自然科学基金(No. 21875196);国家自然科学基金(No. 21303147);国家重点研发计划(No. 2018YFB0905400)
Construction and Electrochemical Performance of Garnet-Type Solid Electrolyte/Al-Li Alloy Interface
Received date: 2018-10-01
Revised date: 2018-11-29
Online published: 2018-11-29
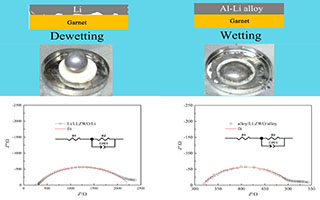
本文通过在锂负极中熔入少量铝制备了一种含Al-Li合金(Al4Li9)的新型复合锂负极,可有效改善Garnet/金属锂界面润湿性,从而显著降低了界面阻抗. XRD研究结果表明这一复合锂负极由Al4Li9合金和金属锂两相复合而成. SEM研究表明,复合锂负极可以有效改善金属锂与Garnet电解质的界面接触,形成更为紧密的接触界面. 电化学测试表明,复合锂负极显著降低了金属锂与Garnet电解质的界面阻抗,界面阻抗由锂/Garnet电解质界面的740.6 Ω·cm 2降低到复合锂负极/Garnet电解质界面的75.0 Ω·cm 2. 使用复合锂负极制备的对称电池在50 μA·cm -2和100 μA·cm -2电流密度锂沉积-溶出过程中表现出较低的极化和良好的循环稳定性,在50 μA·cm -2电流密度下,可以稳定循环超过400小时.
关键词: 石榴石型固体电解质; 电极/固态电解质界面; 铝锂合金
马嘉林 , 王红春 , 龚正良 , 杨勇 . 石榴石型固态电解质/铝锂合金界面构筑及电化学性能[J]. 电化学, 2020 , 26(2) : 262 -269 . DOI: 10.13208/j.electrochem.181001
Garnet-type solid electrolyte is a newly developed Li ion conductor and promising in the application of all-solid-state batteries. However, Garnet is incompatible with Li anode, which restricts the application of Garnet-type solid batteries. In order to improve the contact between Garnet-type solid electrolyte and Li electrode, a composite anode which contains Al-Li alloy (Al4Li9) was prepared as an electrode. Al-Li alloy has many advantages such as easy preparation, low cost, simple post-treatment and high capacity. Garnet-type Li6.5La3Zr1.75W0.25O12 (LLZWO) was synthesized via solid-state reaction. Garnet solid electrolyte has poor interfacial wettability with lithium, but has good interfacial wettability with Al-Li alloy. By using Al-Li alloy as an electrode, the contact between LLZWO and Li electrodes could be well improved. SEM images also confirmed that Al-Li alloy and Garnet had a sufficient interface contact. On the other side, the interface resistance could be dramatically reduced. Impedance spectra show that the interface resistance between Al-Li alloy and Garnet reduced from 740.6 Ω·cm 2 to 75.0 Ω·cm 2, which is only one-tenth of interface resistance between Li alloy and Garnet. Symmetric cell with Al-Li alloy and Garnet showed excellent and stable cycle performance with almost 0 polarization voltage when cycling at a current density between 50 μA·cm -2 and 100 μA·cm -2. At a current density of 50 μA·cm -2, the cell cycled 400 hours stably without formation of lithium dendrite.

/
| 〈 |
|
〉 |