

铂基燃料电池氧还原反应催化剂研究进展
收稿日期: 2018-09-27
修回日期: 2018-11-22
网络出版日期: 2018-11-22
基金资助
国家重点研发计划“新能源汽车”专项(No. 2016YFB0101202)及国家自然科学基金项目(No. 91534205,No. 21436003)资助
Recent Progress in Pt-Based Catalysts for Oxygen Reduction Reaction
Received date: 2018-09-27
Revised date: 2018-11-22
Online published: 2018-11-22
李静 , 冯欣 , 魏子栋 . 铂基燃料电池氧还原反应催化剂研究进展[J]. 电化学, 2018 , 24(6) : 589 -601 . DOI: 10.13208/j.electrochem.180850
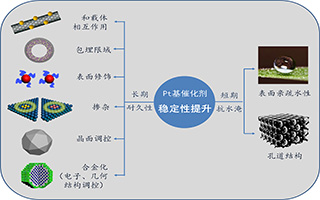
Key words: fuel cells; platinum; alloy; oxygen reduction reaction
/
| 〈 |
|
〉 |