

基于内嵌钴/氮掺杂多孔碳三维石墨烯笼的抗团聚高效氧还原电催化剂
收稿日期: 2018-09-17
修回日期: 2018-10-01
网络出版日期: 2018-11-06
基金资助
国家自然科学基金优秀青年基金(No. 51522203)及面上项目(No. 51772040)、霍英东青年教师基金(No. 151047)与中央高校基本科研业务费(No. DUT18LAB19)资助
Caging Porous Co-N-C Nanocomposites in 3D Graphene as Active and Aggregation-Resistant electrocatalyst for Oxygen Reduction Reaction
Received date: 2018-09-17
Revised date: 2018-10-01
Online published: 2018-11-06
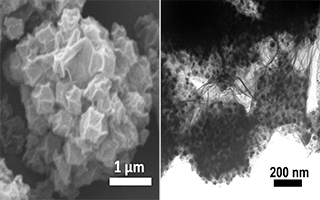
关键词: 氧还原反应;电催化剂;钴/氮掺杂碳; 三维石墨烯;喷雾干燥
修陆洋 , 于梦舟 , 杨鹏举 , 王治宇 , 邱介山 . 基于内嵌钴/氮掺杂多孔碳三维石墨烯笼的抗团聚高效氧还原电催化剂[J]. 电化学, 2018 , 24(6) : 715 -725 . DOI: 10.13208/j.electrochem.180847

/
| 〈 |
|
〉 |