

ZIF-67衍生的Ag/Co@NC材料及其氧还原性能的研究
收稿日期: 2018-07-27
修回日期: 2018-08-28
网络出版日期: 2019-12-28
基金资助
国家自然科学基金(No. 51772097),河北省杰出青年基金(No. E2017209079),河北省自然科学基金(No. B2016109266)
ZIF-67-Derived Ag/Co-Embedded N-Enriched Mesoporous Carbon for Oxygen Reduction Reaction
Received date: 2018-07-27
Revised date: 2018-08-28
Online published: 2019-12-28
Supported by
The project was supported by the Natural Science Foundation of China (No. 51772097), Hebei Natural Science Funds for Distinguished Young Scholar (No. E2017209079) and Hebei Natural Science Fund (No. B2016209266).
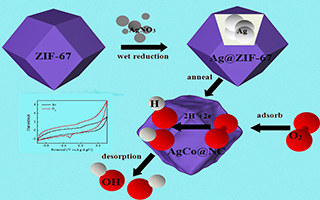
狄正玲 , 朱 靖 , 戴 磊 , 孟 伟 , 李跃华 , 何章兴 , 王 岭 . ZIF-67衍生的Ag/Co@NC材料及其氧还原性能的研究[J]. 电化学, 2019 , 25(6) : 781 -791 . DOI: 10.13208/j.electrochem.180726

Key words: oxygen reduction reaction; ZIF-67; N-doped porous carbon; Ag nanoparticles
/
| 〈 |
|
〉 |