

低铂膜电极结构优化途径
收稿日期: 2018-09-05
修回日期: 2018-09-27
网络出版日期: 2018-10-12
基金资助
国家自然科学基金项目(No. 20833005,No. 20828005,No. 20921120405)资助
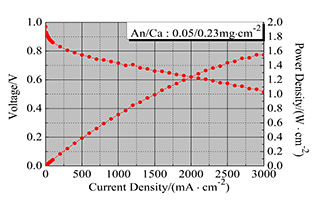
Fuel cell performance curve after MEA optimization Structural Optimization of Low Pt Membrane Electrode Assembly
Received date: 2018-09-05
Revised date: 2018-09-27
Online published: 2018-10-12
饶妍 , 李赏 , 周芬 , 田甜 , 钟青 , 宛朝辉 , 谭金婷 , 潘牧 . 低铂膜电极结构优化途径[J]. 电化学, 2018 , 24(6) : 677 -686 . DOI: 10.13208/j.electrochem.180843

/
| 〈 |
|
〉 |