

酸处理的碳作为阳极电催化剂用于直接抗坏血酸碱性膜燃料电池
收稿日期: 2018-08-27
修回日期: 2018-09-25
网络出版日期: 2018-10-10
基金资助
国家自然科学基金(No.21003114, No.21103163, No.21306188, No.21373211, No. 21306187),国家重点专项(No.2016YFB0101307),辽宁百千万人才计划(No.201519),辽宁省高等学校优秀科技人才支持计划(No.LR201514), 大连市优秀青年科技人才(No.2015R006)资助.
Acid Treated Carbon as Anodic Electrocatalysts toward Direct Ascorbic Acid Alkaline Membrane Fuel Cells
Received date: 2018-08-27
Revised date: 2018-09-25
Online published: 2018-10-10
Supported by
This study was supported by National Key Research & Development Program of China (Gran No. 2016YFB0101307), National Natural Science Fund of China (Grant Nos. 21003114, 21103163, 21306188, 21373211, and 21306187), Liaoning BaiQianWan Talents Program (Grant No. 201519), Program for Liaoning Excellent Talents in University (Grant No. LR201514), Dalian Excellent Young Scientific and Technological Talents (Grant No. 2015R006)
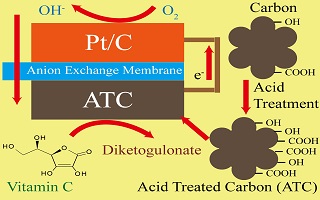
关键词: 直接碱性抗坏血酸燃料电池; 碳; 阳极电催化剂; 活化能; 酸处理
陈禾木, 邱晨曦, 丛媛媛, 刘会园, 翟梓会, 宋玉江 . 酸处理的碳作为阳极电催化剂用于直接抗坏血酸碱性膜燃料电池[J]. 电化学, 2018 , 24(6) : 748 -756 . DOI: 10.13208/j.electrochem.180844

/
| 〈 |
|
〉 |