

Fe-N共掺杂的碳纳米管串联空心球对氧还原反应的电催化
收稿日期: 2018-09-04
修回日期: 2018-09-14
网络出版日期: 2018-09-26
基金资助
黑龙江省自然科学基金(No. QC2013C008)资助
Fe-N Doped Hollow Carbon Nanospheres Linked by Carbon Nanotubes for Oxygen Reduction Reaction
Received date: 2018-09-04
Revised date: 2018-09-14
Online published: 2018-09-26
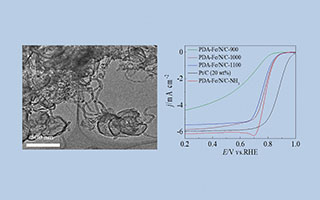
关键词: 非贵金属催化剂; 氧还原反应; 聚多巴胺; 氨气刻蚀; 碳纳米管/空心球复合物
张雅琳 , 陈驰 , 邹亮亮 , 邹志青 , 杨辉 . Fe-N共掺杂的碳纳米管串联空心球对氧还原反应的电催化[J]. 电化学, 2018 , 24(6) : 726 -732 . DOI: 10.13208/j.electrochem.180842

/
| 〈 |
|
〉 |