

金电极上偶氮腺嘌呤的电化学行为研究
收稿日期: 2018-04-01
修回日期: 2018-04-08
网络出版日期: 2019-12-28
基金资助
国家自然科学基金项目(No. 21533006, No. 21621091, No. 21773197)和福建省创新人才资助
Electrochemical Behaviors of Azopurine on Gold Electrodes
Received date: 2018-04-01
Revised date: 2018-04-08
Online published: 2019-12-28
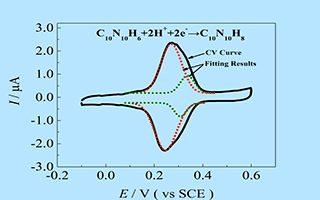
关键词: 偶氮腺嘌呤;表面吸附量;循环伏安法;金电极; 电极动力学
李明雪 , 史 杭 , 刘 佳 , 张 檬 , 周剑章 , 吴德印 , 田中群 . 金电极上偶氮腺嘌呤的电化学行为研究[J]. 电化学, 2019 , 25(6) : 651 -659 . DOI: 10.13208/j.electrochem.180401

/
| 〈 |
|
〉 |