

质子交换膜燃料电池催化层化学稳定性研究
收稿日期: 2017-11-01
修回日期: 2017-11-21
网络出版日期: 2017-12-26
基金资助
国家科技支撑计划项目(No. 2015BAG06B00)和国家重点研发计划(No.2016YFB0101302)资助
Chemical Stability Investigations of Catalyst Layer in PEMFC
Received date: 2017-11-01
Revised date: 2017-11-21
Online published: 2017-12-26
高燕燕 , 侯明 , 姜永燚 , 梁栋 , 艾军 , 郑利民 . 质子交换膜燃料电池催化层化学稳定性研究[J]. 电化学, 2018 , 24(3) : 227 -234 . DOI: 10.13208/j.electrochem.171101
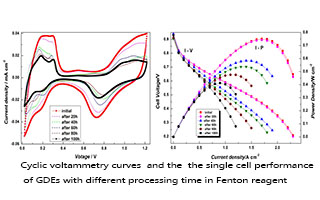
This work was intended to study the effect of free radicals on the chemical stability of catalyst layer via ex situ accelerated stress test (AST). Fenton reagent was used as the free radical provider in this research. Apart from the decomposition of Nafion in catalyst layer, the agglomerated Pt nanoparticles and the corroded carbon support were also observed after being treated in Fenton reagent for 100 h. Firstly, the existence of fluoride (F) ions evidenced the chemical decomposation of Nafion after being attacked by trace radical species, which was supported by the intensity decrease in the C-F stretching and vibration peak (1250 cm-1) observed in attenuated total reflection Fourier transform infrared (ATR-FTIR) spectrum. The transmission electron microscopic (TEM) studies showed that the severe Pt nanoparticles agglomeration and carbon support corrosion happened. To gain more insight, the cyclic voltammetry (CV), ATR-FTIR and single cell performance measurements were conducted. A large loss (58%) of the electrochemical active surface area (ECSA) was observed. Another meaningful finding was the reduced double layer region, which demonstrated the reduction of carbon support. Notably, further ATR-FTIR analysis revealed the disappearance in the spectra at 1719 cm-1 ~ 1578 cm-1 which were assigned to the hydroquinone-quinine (HQ-Q) redox couple on the oxidized carbon surface. On the bases of these results, it could be concluded that carbon support might be decayed by releasing CO2 instead of forming oxides on its surface. Finally, the single cell performance data indicated an obvious performance loss in high current density range, due to the proton and local gas transport limitations in the decayed electrodes.

/
| 〈 |
|
〉 |