

镍离子对中磷镍基体氯化胆碱无氰浸金表面的改善
收稿日期: 2017-04-17
修回日期: 2017-10-19
网络出版日期: 2017-10-20
基金资助
国家自然科学基金(No. 51574047、No. 51601020)项目资助
Surface Ehancement of Nickel Ions on Cyanide Free Immersion Gold Deposited from a Chloroauric Acid-Choline Chloride Solution on Medium-Phosphorus Nickel
Received date: 2017-04-17
Revised date: 2017-10-19
Online published: 2017-10-20
徐天宇 , 王世颖 , 王文昌 , 陈智栋 . 镍离子对中磷镍基体氯化胆碱无氰浸金表面的改善[J]. 电化学, 2018 , 24(1) : 36 -39 . DOI: 10.13208/j.electrochem.170445
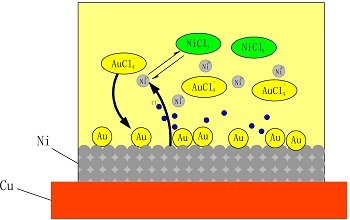
Key words: non-cyanide; immersion gold; choline chloride; hyperactive corrosion; Ni2+
/
| 〈 |
|
〉 |