

可见光诱导提升Pt/g-C3N4纳米片甲酸氧化性能的研究
收稿日期: 2017-06-27
修回日期: 2017-09-20
网络出版日期: 2017-09-29
基金资助
国家自然科学基金项目(No. 21376057,No. 21433003)资助
Pt/g-C3N4 Nanosheet for Visible Light-Induced Enhancement of the Activity for Formic Acid Electro-oxidation
Received date: 2017-06-27
Revised date: 2017-09-20
Online published: 2017-09-29
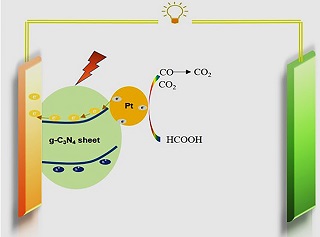
以半导体材料类石墨氮化碳纳米片(g-C3N4纳米片)为载体,通过微波-多元醇法构筑了Pt/g-C3N4纳米片催化剂. 通过TEM、XRD、XPS、紫外-可见吸收光谱等方法对Pt/g-C3N4纳米片催化剂的粒径尺寸、组成、结构、光学等性质进行分析. 通过对比可见光照和暗室条件下的甲酸电氧化活性,Pt/g-C3N4纳米片催化剂在可见光照射下展现出良好的催化性能. 该性能的提高一方面可能是由于g-C3N4纳米片在可见光照射下加速了电子从Pt转移给g-C3N4纳米片,Pt处于“电子匮乏”状态,可削弱CO与Pt之间的化学键能,减弱CO在Pt表面的吸附能力,促进了CO的氧化,提高了催化剂抗中毒能力;另一方面,g-C3N4纳米片在光照条件下分离出的空穴可有效氧化甲酸分子,提高甲酸氧化活性. 因此,可见光条件下可有效提高Pt/g-C3N4纳米片催化剂甲酸催化氧化活性,这为直接甲酸燃料电池的发展提供了新思路.
孙雍荣 , 杜春雨 , 韩国康 , 王雅静 , 高云智 , 尹鸽平 . 可见光诱导提升Pt/g-C3N4纳米片甲酸氧化性能的研究[J]. 电化学, 2018 , 24(3) : 262 -269 . DOI: 10.13208/j.electrochem.170627
By using graphitic carbon nitride nanosheet (g-C3N4 nanosheet) as a support,Pt/g-C3N4 nanosheet catalyst was fabricated by microwave assisted polylol process. The nanoparticles size,composition,structure and optical properties of Pt/g-C3N4 nanosheet were characterized by TEM,XRD,XPS and UV-Vis diffuse reflectance spectroscopy. Comparing with the catalytic activities toward formic acid electro-oxidation under dark and visible light illumination,the superior activity of Pt/g-C3N4 nanosheet catalyst was achieved under visible light illumination. This visible light-driven enhancement in the formic acid performance could be attributed to the plasmon-induced electron-hole separation on g-C3N4 with visible light illumination. The photo-generated hot electron promoted the oxidation of formic acid molecules. The current density of g-C3N4 nanosheet was increased under visible light illumination in the presence of formic acid. More importantly,the fast electron transfer from Pt to g-C3N4 nanosheet under visible light illumination adjusted the electronic structure of Pt. The “electron deficient” state of Pt could weaken the adsorption energy of CO (as a poisoning species),facilitating CO oxidation. The rapid removal of poisoning species on Pt would provide more catalytic activity sites for the oxidation of formic acid,enhancing formic acid catalytic activity. The visible light-assisted enhancement in the electrochemical formic acid activity provides a development strategy for direct formic acid fuel cells.

/
| 〈 |
|
〉 |