

基于碳复合Fe3O4纳米粒子的过氧化氢电化学传感器研究
收稿日期: 2017-05-06
修回日期: 2017-09-09
网络出版日期: 2017-09-17
基金资助
河南省高校科技创新团队支持计划(No. 15IRTSTHN019)资助
Carbon Composite Fe3O4 Nanoparticles Based Electrochemical Sensor for Hydrogen Peroxide Detection
Received date: 2017-05-06
Revised date: 2017-09-09
Online published: 2017-09-17
张思宇 , 王会娟 , 李书芳 , 屈建莹 . 基于碳复合Fe3O4纳米粒子的过氧化氢电化学传感器研究[J]. 电化学, 2018 , 24(3) : 279 -284 . DOI: 10.13208/j.electrochem.170506
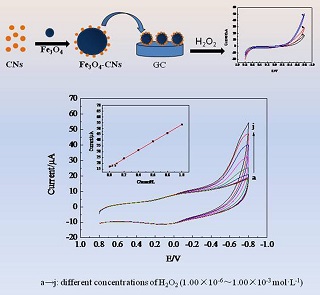
Key words: hydrogen peroxide; Fe3O4 nanoparticles; carbon nanoparticles
/
| 〈 |
|
〉 |