

Co3(HCOO)6@rGO 作为锂离子电池负极材料的研究
收稿日期: 2017-04-12
修回日期: 2017-06-07
网络出版日期: 2017-06-19
基金资助
973 项目(No. 2015CB251102)和国家自然科学基金项目(No. U1305246,No. 21673196,No. 21621091)资助
Co3(HCOO)6@rGO as a Promising Anode for Lithium Ion Batteries
Received date: 2017-04-12
Revised date: 2017-06-07
Online published: 2017-06-19
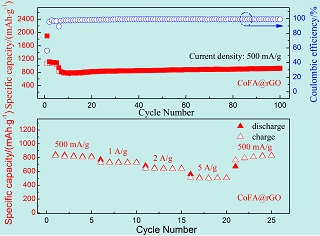
关键词: Co3(HCOO)6@rGO; Co3(HCOO)6; 甲酸根; 负极; 锂离子电池
江恒 , 范镜敏 , 郑明森 , 董全峰 . Co3(HCOO)6@rGO 作为锂离子电池负极材料的研究[J]. 电化学, 2018 , 24(3) : 207 -215 . DOI: 10.13208/j.electrochem.170412

Key words: Co3(HCOO)6@rGO; Co3(HCOO)6; formate ion; anode; lithium ion batteries
/
| 〈 |
|
〉 |