

提高电容性电化学储能装置能量容量的一些尝试
收稿日期: 2017-04-20
修回日期: 2017-07-07
网络出版日期: 2017-10-28
基金资助
浙江省科技计划项目-公益技术应用研究(2017C31104, 2016C31023)和宁波市科技计划(“3315计划”, 2014A35001-1, 2016A610115)资助
Attempts to Improve the Energy Capacity of Capacitive Electrochemical Energy Storage Devices
Received date: 2017-04-20
Revised date: 2017-07-07
Online published: 2017-10-28
Supported by
This work has received funding supports from Ningbo Municipal Government (3315 Plan and IAMET Special Fund, 2014A35001-1, and Ningbo Natural Science Foundation Programme, 2016A610115) and Zhejiang Provincial Applied Research Programme for Commonweal Technology, 2016C31023 and 2017C31104.
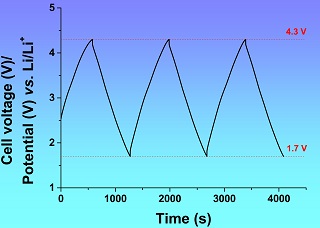
余林颇 , 陈政 . 提高电容性电化学储能装置能量容量的一些尝试[J]. 电化学, 2017 , 23(5) : 533 -547 . DOI: 10.13208/j.electrochem.170347

/
| 〈 |
|
〉 |