

FEC 基电解液对高压正极材料 Li2CoPO4F 电化学性能的影响
收稿日期: 2017-05-11
修回日期: 2017-06-06
网络出版日期: 2017-06-09
基金资助
福建省自然科学基金项目(No.2014J05019)、国家自然科学基金项目(No.21233004,No.21303147)和厦门大学校长基金(No. 20720150090)资助
Influences of FEC-based Electrolyte on Electrochemical Performance of High Voltage Cathode Material Li2CoPO4F
Received date: 2017-05-11
Revised date: 2017-06-06
Online published: 2017-06-09
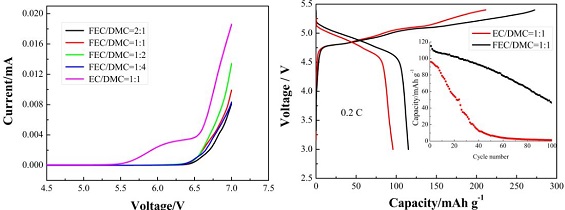
本文研究了以氟代碳酸乙烯酯FEC(fluoroethylene carbonate)为共溶剂的电解液对高压正极材料 Li2CoPO4F 电化学性能的影响,与碳酸酯基电解液1 mol·L-1 LiPF6 EC/DMC=1:1(m:m)相比,1 mol·L-1 LiPF6 FEC/DMC=1:1(m:m)可显著提高Li2CoPO4F的循环稳定性. 通过线性扫描伏安法(LSV)、扫描电镜(SEM)、X射线光电子能谱(XPS)、X射线衍射(XRD)结合电化学阻抗(EIS)对 FEC 改善 Li2CoPO4F 材料循环稳定性的机理进行了探索,结果表明与传统碳酸酯基电解液相比,FEC 基电解液在高压下有着优异的抗氧化性,能够有效抑制电解液的氧化分解. 同时,FEC 基电解液中形成的表面膜具有更高的稳定性,能够抑制电极/电解液界面副反应的发生,提高循环过程中电极材料结构稳定性,从而有益于提高 Li2CoPO4F 材料的电化学性能.
王志刚 , 赵卫民 , 王红春 , 林 敏 , 龚正良 , 杨 勇 . FEC 基电解液对高压正极材料 Li2CoPO4F 电化学性能的影响[J]. 电化学, 2018 , 24(3) : 216 -226 . DOI: 10.13208/j.electrochem.170509
The effects of fluoroethylene carbonate (FEC) as co-solvent on the electrochemical performance of high voltage cathode material Li2CoPO4F are investigated. Compared with traditional carbonate based electrolyte (1 mol·L-1 LiPF6 EC/DMC (1:1, m:m)), the FEC/DMC based electrolyte can significantly improved the electrochemical performance of Li2CoPO4F. After 100 cycles between 3V and 5.4 V at 1 C rate, the capacity retention of Li2CoPO4F electrode in 1 mol·L-1 LiPF6 EC/DMC (1:1, m:m) was 52.6 % , while that in the EC/DMC based electrolyte was only 14.5 %. Possible functional mechanisms of FEC improving the electrochemical performance of Li2CoPO4F were studied by LSV, EIS, SEM and XPS measurements. It was shown that compared with the traditional EC/DMC based electrolyte, the FEC/DMC based electrolyte exhibited higher stability at high voltage, which suppressed the side reactions at electrode/electrolyte interface when charged to high voltage, and improved the structure stability of Li2CoPO4F during cycling, thus, significantly enhanced the electrochemical performance of Li2CoPO4F.

/
| 〈 |
|
〉 |