

水系锌离子电容器
收稿日期: 2017-03-28
修回日期: 2017-05-12
网络出版日期: 2017-10-28
基金资助
国家自然科学基金项目(No. 21601195, 51625204, 21671196)、中国科学院青年创新促进会(No.2017253)、青岛市源头创新计划、青岛市储能行业科学研究智库联合基金及青岛市太阳能与储能技术重点实验室资助
An Aqueous Zn-Ion Capacitor
Received date: 2017-03-28
Revised date: 2017-05-12
Online published: 2017-10-28
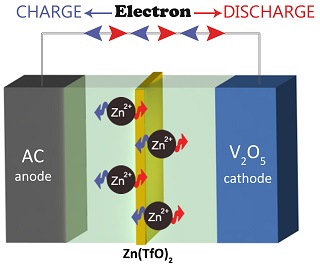
本文首次提出了一种水系锌离子电容器的新型储能体系,其中以五氧化二钒(V2O5)为正极,具有高比表面积的活性炭(AC)为负极,以及三氟甲基磺酸锌(Zn(TfO)2)为电解质. X射线衍射(XRD)证明二价锌离子作为电荷载体,可以在五氧化二钒(V2O5)中进行可逆的嵌入与脱出. 该锌离子电容器的电位窗口可达1.4 V,具有良好的倍率特性及循环稳定性. 电流密度为1000 mA·g-1 时,电容器的比能量密度为4.5 Wh·kg-1,功率密度可达181 W·kg-1. 本工作为发展新型基于多价离子电化学电容器提供了新思路和新方法.
赵井文 , 李佳佳 , 韩鹏献 , 崔光磊 . 水系锌离子电容器[J]. 电化学, 2017 , 23(5) : 581 -585 . DOI: 10.13208/j.electrochem.170344

/
| 〈 |
|
〉 |