

在TiO2纳米阵列上电沉积RuO2用于CO2电还原
收稿日期: 2017-04-07
修回日期: 2017-04-28
网络出版日期: 2017-04-28
基金资助
This work is supported by the 973 Program (No. 2015CB932303) of MOST and NSFC (No. 21473039).
Electrodeposition of RuO2 Layers on TiO2 Nanotube Array toward CO2 Electroreduction
Received date: 2017-04-07
Revised date: 2017-04-28
Online published: 2017-04-28
Supported by
This work is supported by the 973 Program (No. 2015CB932303) of MOST and NSFC (No. 21473039).
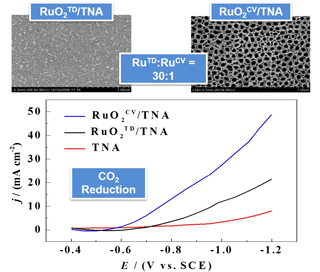
传统上,RuO2/TiO2复合电极制备是通过在TiO2/Ti基体上多次涂覆含Ru前驱体溶液和随后热分解(TD)来实现的. 为克服上述方法中Ru用量大和利用率低之不足, 本工作主要基于循环伏安法(CV)在TiO2纳米管阵列(TNA)上电沉积RuO2制备RuO2CV/TNA复合电极. SEM、GIXRD和CV结果表明, 电沉积的RuO2为无定型结构, 所制备电极中的Ru用量约为传统的RuO2TD/TNA电极中Ru用量的1/30. 尽管两电极催化CO2还原产物的法拉第效率接近, 但是RuO2CV/TNA电极比RuO2TD/TNA电极展示了更高的还原电流, 较正的初始还原电位和更好的稳定性. 与磷酸盐缓冲溶液中电还原CO2相比,RuO2CV/TNA电极在0.1 mol•L-1 KHCO3中电还原CO2除生成更高法拉第效率的甲酸根和甲烷外,还检测到CO的生成.
蒋 孛 , 张莉娜 , 秦先贤 , 蔡文斌 . 在TiO2纳米阵列上电沉积RuO2用于CO2电还原[J]. 电化学, 2017 , 23(2) : 238 -244 . DOI: 10.13208/j.electrochem.161253
RuO2/TiO2 composite materials have multitude of electrocatalytic applications including but not limited to CO2 reduction reaction (CO2RR). RuO2/TiO2 electrodes were previously prepared by repetitive coating and thermal decomposition (TD) of a Ru(III) precursor solution on Ti substrate. In this work, electrochemical potential cycling is applied to deposit amorphous RuO2 (α-RuO2) layers onto TiO2 nanotube array (TNA) (RuO2CV/TNA) preformed on Ti foil. SEM, GIXRD, and voltammetry are applied to characterize the structures of the resulting RuO2CV/TNA. Ru loading on the RuO2CV/TNA electrode is ca. 1/30 of that on the conventional RuO2TD/TNA electrode. Although both electrodes yield similar faradaic efficiencies (FEs) for the reduction products, the RuO2CV/TNA electrode displays a much higher reduction current, a more positive initial reduction potential and a better durability than the RuO2TD/TNA one. In addition to higher FEs for formate and CH4, the RuO2CV/TNA electrode yields the product of CO for the CO2RR in 0.1 mo•lL-1 KHCO3, which is not available in a PBS solution with pH 7.

Key words: CO2 reduction; amorphous RuO2; TiO2 nanotube array; electrodeposition
1. Zhang Z F, Xie E, Li W J, et al. Hydrogenation of carbon dioxide is promoted by a task-specific ionic liquid[J]. Angewandte Chemie International Edition, 2008, 47(6): 1127-1129.
2. Barrosse-Antle L E, Compton R G, Reduction of carbon dioxide in 1-butyl-3-methylimidazolium acetate[J]. Chemical Communications, 2009(25): 3744-3746.
3. Woolerton T W, Sheard S, Reisner E, et al. Efficient and clean photoreduction of CO2 to CO by enzyme-modified TiO2 nanoparticles using visible light[J]. Journal of the American Chemical Society, 2010, 132(7): 2132-2133.
4. Angamuthu R, Byers P, Lutz M, et al. Electrocatalytic CO2 conversion to oxalate by a copper complex[J]. Science, 2010, 327(5963): 313-315.
5. Begum A, Pickup P G, Electrocatalysis of CO2 reduction by ruthenium benzothiazole and bithiazole complexes[J]. Electrochemistry Communications, 2007, 9(10): 2525-2528.
6. Saha M S, Furuta T, Nishiki Y, Conversion of carbon dioxide to peroxycarbonate at boron-doped diamond electrode[J]. Electrochemistry Communications, 2004, 6(2): 201-204.
7. Liu L J, Li Ying, Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review[J]. Aerosol and Air Quality Research, 2014,14(2):453-469
8. Neatu S, Macia-Agullo J A, Garcia H, Solar light photocatalytic CO2 reduction: General considerations and selected bench-mark photocatalysts[J]. International Journal of Molecular Sciences, 2014, 15(4): 5246-5262.
9. Reske R, Duca M, Oezaslan M, et al. Controlling catalytic selectivities during CO2 electroreduction on thin Cu metal overlayers[J]. The Journal of Physical Chemistry Letters, 2013, 4(15): 2410-2413.
10. Zhang S, Kang P, Meyer T J, Nanostructured Tin catalysts for selective electrochemical reduction of carbon dioxide to formate[J]. Journal of the American Chemical Society, 2014, 136(5): 1734-7.
11. Gao D F, Wang J, Wu H H, et al. pH effect on electrocatalytic reduction of CO2 over Pd and Pt nanoparticles[J]. Electrochemistry Communications, 2015, 55, 1-5.
12. Min X Q, Kanan M , Pd-catalyzed electrohydrogenation of carbon dioxide to formate: High mass activity at low overpotential and identification of the deactivation pathway[J]. Journal of American Chemical Sociaty, 2015,137(14):4701-4708.
13. Hori, Y. Electrochemical CO2 reduction on metal electrodes[M]. Modern Aspects of Electrochemistry,Springer: New York, 2008, 42, 89-189.
14. Cheung K C, Guo P, So M H, et al. Electrocatalytic reduction of carbon dioxide by a polymeric film of rhenium tricarbonyl dipyridylamine[J]. Journal of Organometallic Chemistry, 2009, 694(17): 2842-2845.
15. Yano J, Yamasaki S, Pulse-mode electrochemical reduction of carbon dioxide using copper and copper oxide electrodes for selective ethylene formation[J]. Journal of Applied Electrochemistry, 2008, 38(12): 1721-1726.
16. Spataru N, Tokuhiro K, Terashima C, et al. Electrochemical reduction of carbon dioxide at ruthenium dioxide deposited on boron-doped diamond[J]. Journal of Applied Electrochemistry, 2003, 33(12): 1205-1210.
17. Bandi A, Electrochemical Reduction Of Carbon-Dioxide on Conductive Metallic Oxides[J]. Journal of the Electrochemical Society, 1990, 137(7): 2157-2160.
18. Chaplin R P S, Wragg A A, Effects of process conditions and electrode material on reaction pathways for carbon dioxide electroreduction with particular reference to formate formation[J]. Journal of Applied Electrochemistry, 2003, 33(12): 1107-1123.
19. Zhou S H, Eichhorn B W, Jackson G, PtCu core-shell and alloy nanoparticles for NO reduction; Anomalous stability and reactivity[J]. Abstracts of Papers - American Chemical Society, 2005, 230, U2145-U2145.
20. Bandi A, Kuhne H M, Electrochemical reduction of carbon-dioxide in water-analysis of reaction-mechanism on ruthenium-titanium-oxide[J]. Journal of the Electrochemical Society, 1992, 139(6): 1605-1610.
21. Qu J P, Zhang X G, Wang Y G, et al. Electrochemical reduction of CO2 on RuO2/TiO2 nanotubes composite modified Pt electrode[J]. Electrochimica Acta, 2005, 50(16-17): 3576-3580.
22. Popic J ., AvramovIvic M L, Vukovic N B, Reduction of carbon dioxide on ruthenium oxide and modified ruthenium oxide electrodes in 0.5 M NaHCO3[J]. Journal of Electroanalytical Chemistry, 1997, 421(1-2): 105-110.
23. Qin Y H., Yang H H, Lv R L, et al. TiO2 nanotube arrays supported Pd nanoparticles for ethanol electrooxidation in alkaline media[J]. Electrochimica Acta, 2013, 106, 372-377.
24. Liu S Q, Chen A C, Coadsorption of horseradish peroxidase with thionine on TiO2: Nanotubes for biosensing[J]. Langmuir, 2005, 21(18): 8409-8413.
25. Yoo J, Lee K, Schmuki P, Dewetted Au films form a highly active photocatalytic system on TiO2 nanotube-stumps[J]. Electrochemistry Communications, 2013, 34, 351-355.
26. Uddin M T, Nicolas Y, Olivier C, et al. Preparation of RuO2/TiO2 mesoporous heterostructures and rationalization of their enhanced photocatalytic properties by band alignment investigations[J]. The Journal of Physical Chemistry C, 2013, 117(42): 22098-22110.
27. Tian M, Wu G S, Chen A C, Unique electrochemical catalytic behavior of Pt nanoparticles deposited on TiO2 nanotubes[J]. ACS Catalysis, 2012, 2(3): 425-432.
28. Chen B, Hou J B, Lu, K., Formation mechanism of TiO2 nanotubes and their applications in photoelectrochemical water splitting and supercapacitors[J]. Langmuir, 2013, 29(19): 5911-5919.
29. Hu C C, Huang Y H, Cyclic voltammetric deposition of hydrous ruthenium oxide for electrochemical capacitors[J]. Journalof the Electrochemical Society, 1999, 146(7): 2465-2471.
30. Mo Y B, Cai W B, Dong J A, et al. In situ surface enhanced raman scattering of ruthenium dioxide films in acid electrolytes[J]. Electrochemical and Solid State Letters, 2001, 4(9): E37-E38.
/
| 〈 |
|
〉 |