

高首效富镍正极材料LiNi0.6Co0.2Mn0.2O2的合成及电化学性能研究
收稿日期: 2017-03-17
修回日期: 2017-04-20
网络出版日期: 2017-04-24
基金资助
国家自然科学基金项目(No. 21233004, No. 21428303)资助
Synthesis and Electrochemical Properties of Nickel-Rich Cathode Material LiNi0.6Co0.2Mn0.2O2 with High Initial Coulombic Efficiency
Received date: 2017-03-17
Revised date: 2017-04-20
Online published: 2017-04-24
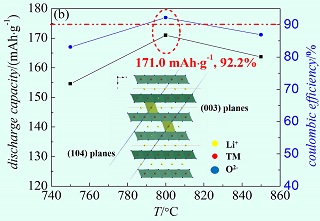
采用共沉淀—高温固相烧结的方法合成了富镍正极材料LiNi0.6Co0.2Mn0.2O2(简称NCM622),通过X射线粉末衍射(XRD)/Rietveld精修法、扫描电子显微镜(SEM)及电化学测试,对不同温度下合成材料的结构、形貌、电化学性能进行表征. 结果表明,800℃下,NCM622阳离子混排程度最低(~1.97%),首圈库伦效率高达92.2%,100圈容量保持率为81.4%.
关键词: 锂离子电池; 正极材料; LiNi0.6Co0.2Mn0.2O2; 首周库仑效率
关小云 , 洪朝钰 , 朱建平 , 王伟立 , 李益孝 , 杨勇 . 高首效富镍正极材料LiNi0.6Co0.2Mn0.2O2的合成及电化学性能研究[J]. 电化学, 2018 , 24(1) : 56 -62 . DOI: 10.13208/j.electrochem.170317
Nickel-rich cathode materials LiNi0.6Co0.2Mn0.2O2(NCM622)were synthesized by a co-precipitation-solid state sintering method at different temperatures. The structure, morphology and electrochemical performance of the as-prepared materials were investigated by X-ray powder diffraction (XRD) /Rietveld refinement, scanning electron microscope (SEM) and electrochemical experiments. It is found that NCM622 calcined at 800 ℃ showed the lowest degree of cation disorder (~1.97%) with a high initial Coulombic effiency of 92.2% and the capacity retention of 81.4% after 100 cycles.

/
| 〈 |
|
〉 |