

N-甲基-N-丁基吡咯烷溴化物和N-甲基-N-乙基吡咯烷溴化物在锌溴液流电池中应用
收稿日期: 2016-10-27
修回日期: 2017-04-13
网络出版日期: 2021-12-17
基金资助
青海省科技支撑计划资助
Applications of N-methyl-N-butyl-pyrrolidinium bromide and N-methyl-N-ethyl-pyrrolidinium bromide in Zn-Br Flow Batteries
Received date: 2016-10-27
Revised date: 2017-04-13
Online published: 2021-12-17
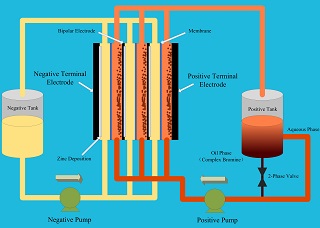
在锌溴液流电池中,作为溴络合剂在电解液中使用的季铵盐对电池性能及安全性具有重要作用.本文利用循环伏安法及线性扫描伏安法研究了N-甲基-N-丁基吡咯烷溴化物(N-methyl-N-butyl-pyrrolidinium bromide,MBP)和N-甲基-N-乙基吡咯烷溴化物(N-methyl-N-ethyl-pyrrolidinium bromide,MEP)分别加入到电解液后对电极反应的影响,通过交流阻抗(EIS)的方式测量了不同组成及浓度下的电解液电导率变化,测试了不同电解液对电池充放电性能的影响及溴络合能力,结果表明加入MBP的电解液具有更好的溴络合能力和电池性能.
关键词: 锌溴液流电池; 季铵盐; N-甲基-N-丁基吡咯烷溴化物; N-甲基-N-乙基吡咯烷溴化物; 络合溴
张祺 , 张苗苗 , 孟琳 . N-甲基-N-丁基吡咯烷溴化物和N-甲基-N-乙基吡咯烷溴化物在锌溴液流电池中应用[J]. 电化学, 2017 , 23(6) : 694 -701 . DOI: 10.13208/j.electrochem.161027

Key words: quaternary ammonium salt; MEP;MBP; Zn-Br flow batteries; bromine complex
/
| 〈 |
|
〉 |