

C-H键选择性直接电氧化研究
收稿日期: 2016-10-31
修回日期: 2017-03-30
网络出版日期: 2017-03-31
基金资助
973前期研究专项(2012CB722604)资助
Selective Direct Electro-Oxidation of C-H Bond
Received date: 2016-10-31
Revised date: 2017-03-30
Online published: 2017-03-31
廖艳梅 , 武倩倩 , 张安伦 , 朱英红 , 马淳安 . C-H键选择性直接电氧化研究[J]. 电化学, 2017 , 23(3) : 276 -282 . DOI: 10.13208/j.electrochem.161046
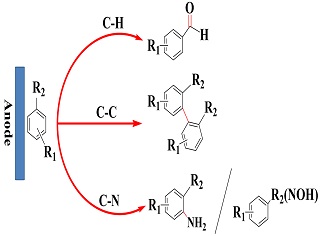
Key words: C-H bond; direct electro-oxidation; selectivity
[1]Herrías C I, Yao X Q, Li Z P, et al. Reactions of C-H bonds in water[J]. Chemical Reviews, 2007, 107: 2546-2562.
[2]Bergman R G. C-H activation[J]. Nature, 2007, 466: 391-393.
[3]Skouta R and Li C J. Gold-catalyzed reactions of C-H bonds[J]. Tetrahedron, 2008, 64: 4917-4938.
[4]Yoo E J, Ma S, Mei T S, et al. Pd-catalyzed intermolecular C-H amination with alkylamines[J]. Journal of The American Chemical Society, 2011, 133(20): 7652-7655.
[5]Sun C L, Li B J, Shi Z J. Direct C-H transformation via iron catalysis[J]. Chemical Reviews, 2011, 111: 1293-1314.
[6]McMurray L, O’Hara F and Gaunt M J. Recent developments in natural product synthesis using meta-catalysed C-H bond functionalisation[J]. Chemical Society Reviews, 2011, 40: 1885-1898.
[7]Liao K B, Negretti S, Musaev D G, et al. Site selective and stereoselective functionalization of unactivated C-H bonds[J]. Nature, 2016, 533: 230-234.
[8] Horn E J, Rosen B R, Chen Y, et al. Scalable and sustainable electrochemical allylic C-H oxidation[J]. Nature, 2016, 533:77-81.
[9] Díaz-Requejo M M, Pérez P J. Coinage metal catalyzed C− H bond functionalization of hydrocarbons[J]. Chemical reviews, 2008, 108(8): 3379-3394.
[10]Giri R, Shi B F, Engle K M, et al. Transition metal-catalyzed C-H activation reactions: diastereoselectivity and enantioselectivity[J]. Chemical Society Reviews, 2009, 38(11): 3242-3272.
[11]Ma C A(马淳安). Introduction to organic electrosynthesis(有机电化学合成导论)[M]. Beijing: Science Press(科学出版社), 2002.
[12]Ogawa K A and Boydston A J. Recent developments in organocatalyzed electroorganic chemistry[J].Chemistry Letter, 2015, 44: 10-16.
[13]Horn E J, Rosen, B R, Chen Y, et al. Scalable and sustainable electrochemical allylic C-H oxidation[J]. Nature, 2016, 533: 77-81.
[14]Sequeira C A C and Santos D M E. Electrochemical routes for industrial synthesis[J]. Journal of Brazilian Chemistry Society, 2009, 20(3):387-406.
[15]Frontana-Uribe B A, Little R D, Lbanez J G, et al. Organic electrosynthesis: a promising green methodology in organic chemistry[J]. Green Chemistry, 2010, 12: 2099-2119.
[16]Moeller K D. Synthtic applications of anodic electrochemistry[J]. Tetrehedron, 2000, 56:9527-9544.
[17]Dudkina Y B, Gryaznova T V, Sinyashin O G, et al. Ligand-directed electrochemical functionalization of C-H bonds in the presence of the palladium and nickel compounds[J]. Russian Chemical Bulletin, 2015, 64(8):1713-1725.
[18]Yoshida J I, Kataoka K, Horcajada R, et al. Modern strategies in electroorganic synthesis[J]. Chemical Reviews, 2008, 108: 2265-2299.
[19]Wu L L(吴玲玲), Zhu Y H(朱英红), L S S(李姗姗), et al. Study on the direct electo-oxidation of anisaldehyde[J]. Journal of Electrochemistry(电化学), 2011, 17(2): 227-230.
[20]Zhu Y H(朱英红), Zeng H Y(曾红燕), Li S S(李姗姗), et al. Electrochemical oxidation of p-methoxy toluene in BMIBF4 ionic liquid[J]. Acta Physico-Chimica Sinica(物理化学学报), 2012, 28(2): 421-426.
[21]Zhu Y H, Zhu Y, Zeng H Y, et al. A promising electro-oxidation of methyl-substituted aromatic compounds to aldehydes in aqueous imidazole ionic liquid solution[J]. Journal of Electroanalytical Chemistry, 2015, 751: 105-110.
[22]Chen Q(陈琼), Zhu Y H(朱英红), Zhu Y(朱颖), et al. Effect of imidazaole ionic liquids on the electo-oxidation of p-methoxy toluene[J]. Journal of Electrochemistry(电化学), 2014, 20(5):465-469.
[23]Zhu Y H, Chen Z Y, Zhang J Q, et al. The activation of C-H bonds using an EmimAc/MWCNTs composite: a comparison of the composite used as electrolyte and electrode in aqueous media[J], Electrochimica Acta, 2016, 207:308-312.
[24]Meng L, Su J H, Zha Z G, et al. Direct electrosynthesis of ketones from benzylic methylenes by electrooxidative C-H activation[J]. Chemistry A European Journal, 2013, 19:5542-5545.
[25]Racowski J M, Ball N D and Sanford M S. C-H bond activation at palladium(Ⅳ) centers[J]. Journal of The American Chemical Society, 2011,133: 18022-18025.
[26]Satio F, Aiso H and Kochi T, et a1.Palladium-catalyzed regioselective homocoupling of arenes using anodic: oxidation formal electrolysis of aromatic carbon-hydrogen bonds[J]. Organometallics, 2014, 33: 6704-6707.
[27]Kirste A, Nieger M, Malkowsky I M, et al. Ortho-selective phenol-coupling reaction by anodic treatment on boron doped diamond electrode using fluorinated alcohols[J]. Chemistry A European Journal, 2009,15: 2273-2277.
[28]Morofuji T, Shimizu, A and Yoshida J I. Metal and chemical-oxidant-free C-H/C-H cross-coupling of aromatic compounds the use of radical-cation pools[J]. Angewandte Chemine International Edition, 2012, 51: 7259-7262.
[29]Morofuji T, Shimizu A and Yoshida J I. Electrochemical C-H amination synthesis of aromatic primary amines via n-arylpyridinium ions[J]. Journal of The American Chemical Society, 2013, 135: 5000-5003.
[30]Herold S, Möhle S, Zirbes M, et al. Electrochemical amination of less-activated alkylated arenes using boron-doped diamond anodes[J].European Journal of Organic Chemistry, 2016, 7: 1274-1278.
[31]Zhang L, Chen H, Zha Z G, et al. Electrochemical tandem synthesis of oximes from alcohols using KNO3 as the nitrogen source, mediated by tin microspheres in aqueous medium[J]. Chemical Communications, 2012, 48: 6574-6576.
/
| 〈 |
|
〉 |