

酸性溶液中Fe2+ /Fe3+相互转换对电极还原行为的影响
收稿日期: 2016-11-29
修回日期: 2017-03-03
网络出版日期: 2017-03-30
基金资助
广西科技计划基地和人才专项(重点实验室建设项目, No.16-380-35):资助
Effect of Fe2+/Fe3+Interconvertion on Reduction Behavior of Fe3+ in Acidic Electrolytes
Received date: 2016-11-29
Revised date: 2017-03-03
Online published: 2017-03-30
采用线性扫描伏安法和循环伏安法研究了含有Cl-、SO42-的酸性溶液中Fe2+/Fe3+相互转换对电极反应和Fe3+还原过程的影响. 结果表明: [Fe]T=1 mol·L-1条件下,溶液中Fe3+/Fe2+的还原析出过程经过两个阶段:(1) E=0.35 V左右Fe3+还原成Fe2+过程; (2) E=-0.3 V之后H+的还原同时Fe2+离子与OH-相结合生产Fe(OH)2; Fe3+/Fe2+的相互转化主要影响Fe3+的第一还原阶段的还原峰电流和峰电位. |ipa/ipc|值随c(Fe3+ )/c(Fe2+ )增大而增大,且扫描速度慢时影响大,扫描速度快时影响小; 0.50 mol·L-1 Fe2++0.50 mol·L-1 Fe3+时,随扫描速率的变化|ipa/ipc|值变化最小(|ipa/ipc|≈1.20). 同时,c(Fe3+ )/c(Fe2+ )也影响平衡电位,平衡电位随c(Fe3+ )/c(Fe2+ )增大而正移,电位从E1=0.501 V升至E5=0.565 V.
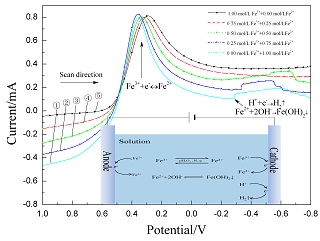
关键词: Fe2+/Fe3+相互转换; 循环伏安; 极化曲线; 还原行为
秦建新 , 林 峰 , 刘文平 , 陈 超 , 任孟德 . 酸性溶液中Fe2+ /Fe3+相互转换对电极还原行为的影响[J]. 电化学, 2017 , 23(6) : 702 -707 . DOI: 10.13208/j.electrochem.161129

/
| 〈 |
|
〉 |