

掺氮碳化钨的制备及其电催化性能的研究
收稿日期: 2017-02-22
修回日期: 2017-03-28
网络出版日期: 2017-03-30
基金资助
国家自然科学基金(No. 21376220);浙江省自然科学基金(No. LY16B060009, No. LY12B03008)资助
Preparation and Electrocatalytic Activity of Nitrogen-Doping Tungsten Carbide Catalyst
Received date: 2017-02-22
Revised date: 2017-03-28
Online published: 2017-03-30
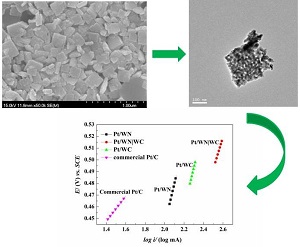
碳化钨是一种具有应用前景的电催化剂,本文尝试对碳化钨的非金属位进行氮掺杂,以钨酸钠为钨源,经由中间体氮化钨(WN),并在一氧化碳气体中进行渗碳后合成掺氮的碳化钨纳米片(WN|WC). 通过扫描电镜(SEM)和透射电镜(TEM)观测发现,WN|WC纳米片尺寸均匀,碳原子进入WN晶格中形成具有密排六方结构的WC晶相,并和WN的晶格条纹紧密联结而形成异质结构. X射线衍射(XRD)结果显示碳化后的样品中含有WN和WC两种晶型,XPS结果进一步表明WN|WC表面形成了WN和WC的异质结构. 为讨论氮元素掺杂对电催化性能的影响,本文通过微波辅助加热法负载少量铂制备Pt/WN|WC催化剂,并以甲醇氧化为指针反应,纯相碳化钨和商用铂碳材料(Pt/C)等为对比样,评价了Pt/WN|WC催化剂的电化学性能. 电化学测试表明,该催化剂甲醇氧化的电流密度是商业Pt/C的3倍,具有较高的交换电流密度和速率常数,且经过200周的循环伏安扫描后,正扫峰电位(Epf)和负扫峰电位(Epb)仍保持稳定,结果表明氮的掺杂改变了碳化钨表面的电子状态,形成了WN和WC的异质界面,有利于催化性能的提高.
杨翩翩 , 黄丽珍 , 李影影 , 施梅勤 , 马淳安 . 掺氮碳化钨的制备及其电催化性能的研究[J]. 电化学, 2018 , 24(1) : 63 -71 . DOI: 10.13208/j.electrochem.170222
Tungsten carbide (WC) is a promising electrocatalyst, however, its electrocatalytic activity is far inferior to Pt and Pt-group metal. In this work, nitrogen-doping tungsten carbide (WN|WC) catalysts with a nanoplate morphology were prepared via the tungsten nitride (WN) as the precursor and sodium tungstate as the tungsten source. The SEM and TEM results indicated that carbon atoms entered into the WN lattice to form the hexagonal close packed WC phase. In this way, an atomic scale heterostructure involving the closely linked interfaces between WN and WC was created . The XRD data confirmed that the cubic crystal structure of WN was still reserved after carbonization. The XPS analyses also verified the existences of W-N and W-C. In order to discuss the effect of nitrogen doping on the catalytic performance of WN|WC, Pt/WN|WC catalyst was prepared by ethylene glycol reduction with microwave-assisted heating method. The electrochemical properties of Pt/WN|WC catalyst in methanol oxidation reaction were evaluated and compared with those of pure WC and commercial Pt/C catalysts. Electrochemical tests demonstrated that the peak current density of Pt/WN|WC was three times as that of commercial Pt/C. In addition, the excellent exchange current density, rate constant and the stable Epf and Epb values suggested that the nanoplate Pt/WN|WC has a promising application as an anode material for direct methanol fuel cells.

/
| 〈 |
|
〉 |